The FDA has cleared Neurophet AQUA AD Plus through the 510(k) pathway. The software supports neuroimaging analysis in Alzheimer’s disease and is Neurophet’s third FDA-cleared product in the US. Previously, the company received FDA 510(k) clearance for Neurophet AQUA and Neurophet SCALE PET.
Neurophet is a South Korea-based company that develops AI-based software for brain imaging analysis and neurological disorders.
Before receiving FDA clearance, Neurophet AQUA AD Plus was designated an Innovative Medical Technology in South Korea in September 2025, a classification used to support early clinical adoption of certain medical technologies within the country.
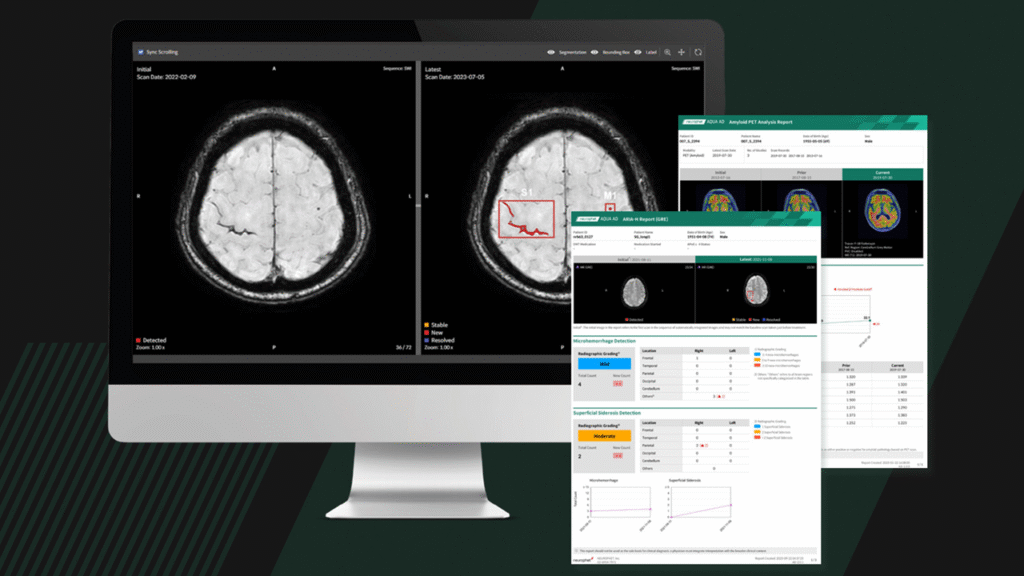
Neurophet AQUA AD Plus is a software used to analyze brain MRI and PET scans that have already been obtained as part of routine clinical care. It generates quantitative imaging outputs that clinicians can review alongside standard image interpretation and other clinical information.
Using AI-based analysis, the software automatically labels brain structures, measures regional brain volumes and identifies specific lesion types visible on MRI. For PET imaging, it calculates standardized uptake value ratios (SUVRs), which are commonly used to quantify tracer uptake in the brain. These results are presented as standardized measurements and visual overlays to support imaging-based assessment.
The FDA-cleared US version of AQUA AD Plus expands on Neurophet’s earlier AQUA AD platform by adding automated analysis of specific MRI-detected lesions relevant to Alzheimer’s disease evaluation. Using AI-based brain MRI analysis, the software identifies and quantifies hypointense lesions associated with cerebral microbleeds and superficial siderosis, as well as hyperintense lesions linked to brain edema.
These imaging findings are relevant to imaging-based assessment during diagnosis, monitoring and treatment planning in Alzheimer’s disease. By automatically localizing lesions and providing lesion counts, the software generates standardized quantitative outputs that can be reviewed alongside routine clinical image interpretation.
Neurophet AQUA AD Plus integrates MRI and PET analysis within a single software environment. This allows clinicians to review structural MRI findings alongside quantitative PET measures when assessing Alzheimer’s disease-related imaging data.
Jake Junkil Been, Co-CEO of Neurophet, indicated that the company plans to expand collaboration with healthcare institutions and partners as part of its US rollout.
The company plans to continue expanding clinical adoption of the software in additional global healthcare markets, focusing on AI-supported neuroimaging tools for brain disorders.
Thursday, March 12, 2026, at 11am EDT (4pm CET/EU-Central)
Register for this webinar to learn how product safety compliance applies to connected medical devices and the testing pathways that support market access.
Recent Studies in AI-Based Brain Imaging and Assessment
AI-based medical imaging is being evaluated across different areas of neurological care. These findings reflect ongoing research into earlier neurological risk assessment.
In December 2025, UK-based Brainomix reported real-world evidence showing that use of its Brainomix 360 Stroke imaging decision-support software across NHS hospitals in England was associated with higher rates of endovascular thrombectomy. The study also reported reduced delays in patient triage and transfer, particularly in non-specialist hospitals.
In January 2026, US-based Hyperfine reported clinical data indicating that its FDA-cleared portable MRI system, combined with AI-enabled image analysis, improved the detection of small ischemic stroke lesions. The data also showed reduced scan times in evaluated settings, including emergency and bedside environments.
Separately, BrainScope, a US-based neurotechnology company, reported peer-reviewed findings showing that its AI-enabled EEG biomarker could identify individuals at risk of future cognitive decline several years before clinical diagnosis.
Outside of research, Belgium-based icometrix announced a collaboration with AstraZeneca and the Middle East North Africa Committee for Treatment and Research in Multiple Sclerosis (MENACTRIMS), a regional clinical research network. The initiative applies AI-enabled MRI analysis to support assessment of neuromyelitis optica spectrum disorder (NMOSD), a rare autoimmune disorder affecting the central nervous system, across the Middle East and Africa.
The initiative will analyze MRI data from the MENACTRIMS registry, including approximately 300 patients across 16 clinical sites, aiming to improve differentiation between NMOSD and multiple sclerosis.
If you want your company to be featured on Xtalks.com, please email [email protected].













Join or login to leave a comment
JOIN LOGIN