Following an alarming number of device recalls, the US Food and Drug Administration (FDA) has beefed up its medical device adverse event reporting procedures. But despite efforts to simplify this process and increase public access to these documents, device users may not become aware of this information until it’s too late.
Charlie Kim, a father and business leader from the Boston area, knows first-hand the consequences of being kept in the dark. His daughter nearly died from a medical device failure as a result of him not knowing that the same device was recalled earlier that year.
“I’ve experienced first-hand what it feels like to wonder if a medical device that your loved one uses—relies on—is safe,” said Kim. “It’s a feeling that no patient, parent or caregiver should have to endure.”

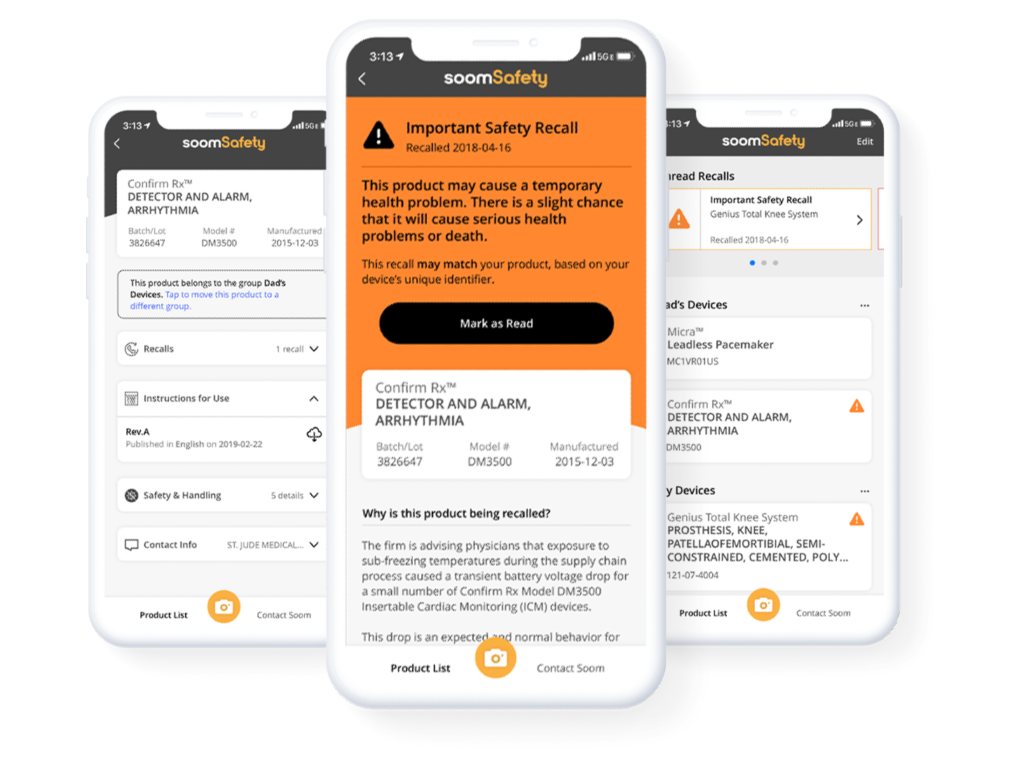
Knowing that dozens of families could lose loved ones over information gaps, he formed Soom, a Software-as-a-Service (SaaS) company, devoted to delivering healthcare information to those who need it. Yesterday, the company launched its SoomSafety mobile app, which connects device users to product data and recall notifications as they arise.
The FDA has its own MedWatcher app which enables patients and clinicians to submit adverse events reports using a smartphone or tablet, but this doesn’t connect these reports to those who may be affected.
“We built SoomSafety to help patients and caregivers relying on implanted medical devices and using medical devices at home answer one critical question, ‘Is this medical device safe to use?’” said Kim in a statement. “Our technology makes it possible to connect previously siloed medical device data, giving patients—and their caregivers—more proactive control over their health and safety.”
The app pulls data from openFDA, a publicly available database containing adverse events reports and product information. To access information about their inhalers, insulin pumps or pacemakers, users scan the device’s unique barcode. Recall information about an exact medical device is often difficult to find, thus the device barcode feature eliminates the need to sift through irrelevant information.
At the same time, it could be helpful for users to be aware of incidents that could potentially affect their medical device. For example, device manufacturers could still be feeling the aftereffects of the Sterigenics facility closure in April, which left many devices unsterilized. To date, 26 medical devices have been recalled by the FDA.
Kim hopes that the SoomSafety app will empower patients, caregivers and nurses with easy to access, immediate answers and actionable information. He tells Xtalks that further developments for the SoomSafety app are underway.
“Future releases of SoomSafety will allow for more direct to consumer interaction between patients and medical device manufactures. Additionally, SoomSafety will be expanded to include pharmaceutical drug safety and recall information.”










Join or login to leave a comment
JOIN LOGIN