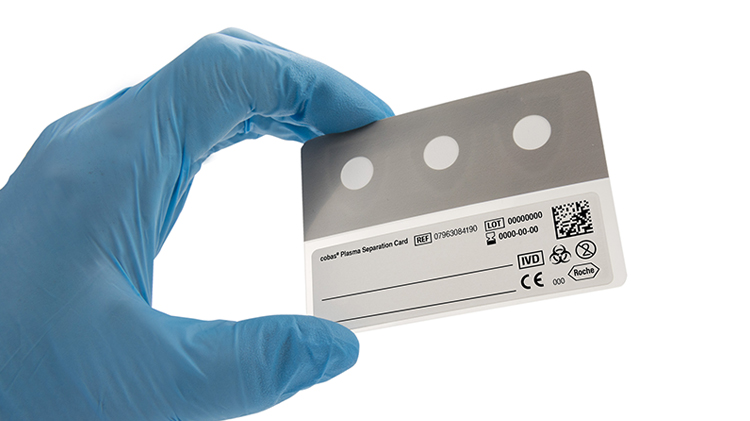
Roche’s new cobas Plasma Separation Card solves some sample collection and storage issues inherent in current devices for HIV testing. The device easily and accurately quantitates HIV viral load from fingertip blood samples.
“With the launch of the cobas Plasma Separation Card, we strengthen Roche’s ongoing commitment to providing life-saving diagnostics in the fight against HIV and AIDS,” said Roland Diggelmann, CEO Roche Diagnostics. “This card will improve access and increase HIV diagnostics scaling up efforts to further the critical work of our many healthcare partners in eradicating the HIV/AIDS epidemic.”
Other plasma collection devices require blood samples to be held in a cool environment while on route to the testing lab. By changing the way plasma samples are collected from patients and further processed, Roche’s cobas Plasma Separation Card keeps samples stable, even in hot and humid environments.
“The cobas Plasma Separation Card is a very impressive innovation,” said Alan Staple, Head of Global Markets Team at The Clinton Health Access Initiative. “Coupled with the successful Global Access Program, this will make a significant contribution to extending both the reach and accuracy of HIV viral load testing for underserved populations.”
Roche’s collection card is a CE-marked device which meets WHO requirements for sensitivity. In addition to helping patients identify their HIV status, it can also support continued patient monitoring efforts to determine whether antiretroviral therapies are keeping the virus in check.
Through Roche’s Global Access Program, the company has been helping to expand access to HIV diagnostics in areas where the virus in endemic. The Joint United Nations Programme on HIV/AIDS (UNAIDS), the Clinton Health Access Initiative (CHAI), Unitaid, the US President’s Emergency Plan For AIDS Relief (PEPFAR) and the Global Fund to fight AIDS, TB and Malaria, have all partnered with Roche to support this goal.











Join or login to leave a comment
JOIN LOGIN