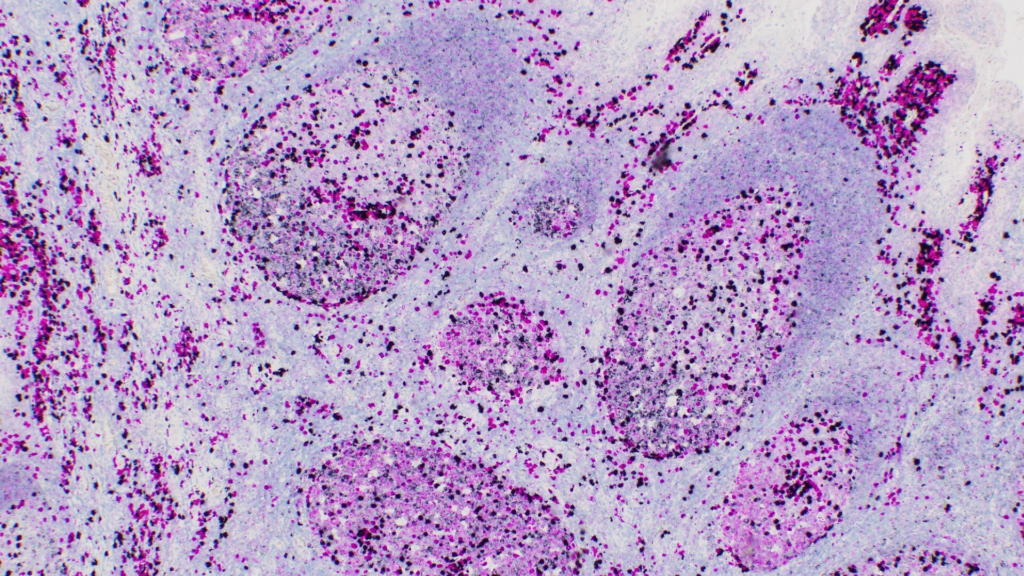
Roche has announced the FDA clearance of its VENTANA Kappa and Lambda Dual in-situ hybridisation (ISH) mRNA Probe Cocktail assay, which is designed to aid in diagnosing B-cell lymphoma, a cancer that develops in white blood cells — specifically B lymphocytes or B cells — of the immune system.
The test, the first of its kind, offers a highly sensitive method to differentiate between a B-cell malignancy and a reactive immune response. It is particularly valuable in cases where biopsy results are inconclusive, providing clarity in often elusive, traditional diagnostic workflows.
The VENTANA assay’s ability to analyze over 60 B-cell lymphoma subtypes equips pathologists with the tools to make faster, more informed decisions. Its compatibility with formalin-fixed tissues not only simplifies logistics but also enables widespread application in hospitals and labs worldwide.
Diagnosing B-cell lymphoma — which accounts for 85% of non-Hodgkin lymphoma cases — is fraught with challenges arising from both biological variability and practical constraints. Subtypes like activated B-cell and germinal center B-cell lymphoma exhibit distinct molecular profiles that complicate standard diagnostic approaches.
XTALKS WEBINAR: Enhance Early Biomarker Discovery: Translating In Vitro and In Vivo Multi-Omics Oncology Data
Live and On-Demand: Thursday, January 16, 2025, at 11am EST (4pm GMT/UK)
Register for this free webinar to learn how to drive early biomarker discovery by harnessing the full potential of multi-omics data and advanced patient models.
Disease symptoms such as lymph node swelling or fatigue frequently mimic those of infections, adding to the uncertainty.
Diffuse large B-cell lymphoma (DLBCL), the most prevalent and aggressive subtype, is further complicated by high relapse rates, particularly in older adults, where over 40% of cases become refractory or relapsed. Advanced age, often a factor at diagnosis, magnifies these difficulties with reduced treatment tolerance and the aggressive nature of certain subtypes.
In some settings, limited access to advanced imaging tools, incomplete immunohistochemistry and inconsistent record-keeping further hinder accurate assessments, often delaying treatment.
The VENTANA Kappa and Lambda Dual ISH assay could address these gaps by evaluating kappa and lambda light chains — key markers on B-cells that reveal whether the cells are part of a reactive immune response or a malignancy. By allowing simultaneous assessment of these markers on a single tissue slide, the test conserves precious tissue samples, reduces the need for repeat biopsies and streamlines workflows for pathologists.
With the FDA clearance, Roche takes a pivotal step in expanding its hematopathology portfolio, which already includes over 65 diagnostic biomarkers.
Jill German, head of Roche Diagnostics’ pathology lab, is hopeful that the VENTANA assay will alleviate the diagnosis of complex B-cell lymphoma cases while simplifying workflows for pathologists.
“Accurately differentiating lymphoma from an infection is critical in ensuring accurate and timely diagnosis, especially as the symptoms can appear similar,” said German in the press release. “With this new test, clinicians can have confidence in their diagnosis, while the test reduces the need for multiple samples and time consuming follow up tests, giving patients certainty sooner and enabling faster access to the right treatment.”









Join or login to leave a comment
JOIN LOGIN