Update (August 24, 2018): In an effort to help patients deal with the ongoing EpiPen shortage, the FDA has extended the expiration date of specific lots of the device by four months. Their hope is that this will give patients whose EpiPens are nearing expiry, or have already expired, more time in which to potentially use the auto-injector while Mylan resolves its supply issues.
Originally published on August 20, 2018:
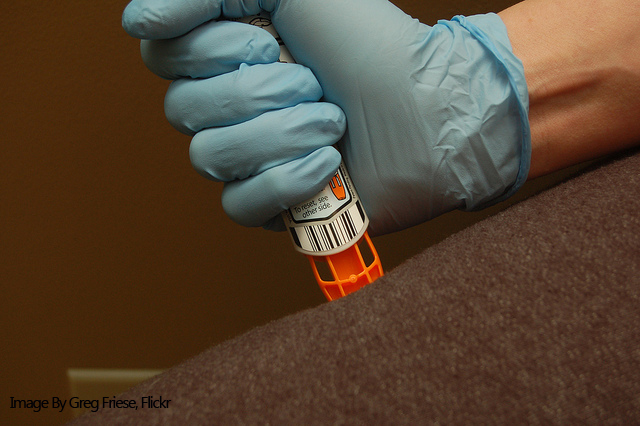
It’s been a long time coming, but US patients will finally have access to the first generic version of the EpiPen, developed by Israeli pharmaceutical company Teva. The US Food and Drug Administration (FDA) approved Teva’s generic versions of the EpiPen and EpiPen Jr and the timing couldn’t be better for patients as Mylan has been struggling to provide adequate supply of the auto-injector to pharmacies in the US and Canada.
“Today’s approval of the first generic version of the most-widely prescribed epinephrine auto-injector in the US is part of our longstanding commitment to advance access to lower cost, safe and effective generic alternatives once patents and other exclusivities no longer prevent approval,” said FDA Commissioner Scott Gottlieb. “This approval means patients living with severe allergies who require constant access to life-saving epinephrine should have a lower-cost option, as well as another approved product to help protect against potential drug shortages.”
The EpiPen shortage has been affecting Canadian patients since the beginning of the year, with the manufacturing issues eventually impacting US supply starting in May. Mylan’s EpiPen is produced by Meridian Medical, a Pfizer-owned manufacturer, which has been facing manufacturing delays, potentially as a result of a component provided by a third-party supplier.
“Today’s approval marks an important step forward in bringing patients an additional epinephrine auto-injector option and furthers our commitment to accessibility to quality products for the patients who need them,” said Brendan O’Grady, Executive Vice President, North America Commercial at Teva Pharmaceuticals.
While Teva also commented that it will be launching the generic EpiPen in the coming months, the drugmaker has yet to release any pricing information for the combination device. The cash price for a two-pack of Mylan’s own authorized generic EpiPen is around $300 – making it more than 50 percent less expensive than its branded counterpart – however this device has also been affected by manufacturing problems that have impacted supply. This means that Teva’s newly-approved generic EpiPen may not even need to beat this list price in order to gain substantial market share.
RELATED: Kaléo Reaffirms Availability of EpiPen Rival While Mylan Struggles with Manufacturing Issues
While Teva was responsible for getting its Abbreviated New Drug Application (ANDA) for the generic EpiPen approved by the FDA, the company partnered with New Jersey-based Antares Pharma in order to use its VIBEX device to deliver the epinephrine injection. The deal also stipulates that Antares is responsible for manufacturing the combination product, while Teva will handle the commercialization and distribution of the generic EpiPen devices.
“We are extremely pleased with the FDA’s decision to approve Teva’s ANDA for the first and only fully substitutable generic version of Mylan’s branded EpiPen, the most widely used epinephrine auto-injector on the market,” said Robert F. Apple, President and Chief Executive Officer of Antares Pharma. “We and our partner Teva worked diligently together to obtain the approval of this very complex drug/device rescue pen combination product utilizing our VIBEX auto-injector platform and we look forward to Teva making it commercially available to patients.”
Antares has a special focus on the auto-injector market, recently announcing a partnership with pharma giant Pfizer to develop a combination device. However, the two companies are keeping the details of the device under wraps, only disclosing that the auto-injector will be used to administer “an undisclosed Pfizer drug.”
Last week, Kaléo released a statement reassuring patients that its EpiPen rival, the AUVI-Q, is not affected by the ongoing manufacturing problems experienced by Mylan. As such, the company confirmed that the device is widely available in the US and that it “has sufficient supply to meet any anticipated demand.”






Join or login to leave a comment
JOIN LOGIN