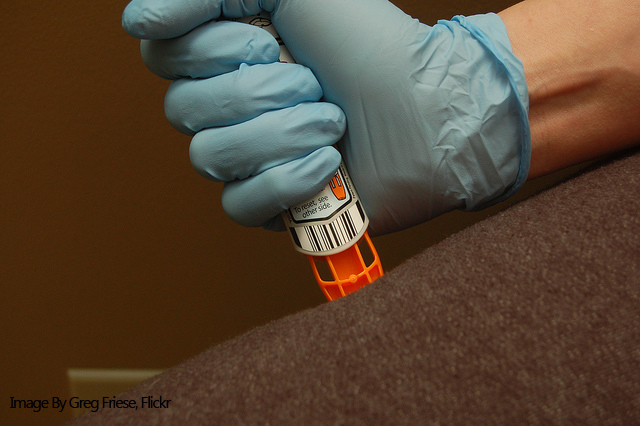
Kaléo is poised to make their mark on the US epinephrine auto-injector market as the company has announced their AUVI-Q device continues to be available while other manufacturers face shortages. In a recent press release, Kaléo reaffirmed that the company “has sufficient supply to meet any anticipated demand.”
Kaléo’s announcement comes as Mylan has struggled with manufacturing issues involving their best-selling EpiPen auto-injectors, which are now affecting supply of the life-saving drug device combination product in the US. The epinephrine auto-injectors are manufactured for Mylan by Meridian Medical, a Pfizer-owned subsidiary, which has been having problems making the devices for other markets since the beginning of the year.
“Over the past few months, there has been intermittent supply of EpiPen at wholesalers and pharmacies,” said a recent statement issued by Mylan. “We are actively exploring several options with Pfizer that would help stabilize supply. Pfizer is working hard to increase production and stabilize supplies, but until this occurs, supplies of EpiPen 0.3 mg and EpiPen Jr 0.15 mg auto-injectors, and the authorized generic versions of these strengths, will continue to vary from pharmacy to pharmacy and may not always be available.”
EpiPen Shortage in Canada Still Affecting Supply
While the US Food and Drug Administration (FDA) lists both versions of the EpiPen as available as of July 31 on its FDA Drug Shortages page, it also lists “manufacturing delays” as the reason behind recent supply issues.
Like the EpiPen, AUVI-Q is approved by the FDA to treat anaphylaxis and other life-threatening allergic reactions through an auto-injected dose of epinephrine. Unique to the AUVI-Q is its method of epinephrine delivery, which utilizes an auto-retractable needle system that pulls the needle tip back into the device after the active ingredient has been injected.
“We understand how critically important it is for those affected by life-threatening allergies to be able to access an epinephrine auto-injector, especially as families prepare for the back to school season. Kaléo is able to fill, and is filling, all the AUVI-Q orders through our Direct Delivery service at www.auvi-q.com,” said Phil Rackliffe, General Manager of Allergy and Pediatrics, at Kaléo. “It’s important to note that patients must obtain AUVI-Q through the Direct Delivery service to ensure delivery to their home or healthcare provider’s office and the best expiration dating for AUVI-Q.”
It’s been over half a year since Canadian patients have had reliable access to the EpiPen, with Mylan citing continuing manufacturing issues which are unlikely to be resolved by the end of August. In May, the company extended their shortage to the US.
In 2017, Kaléo set the list price for a two-pack of AUVI-Q auto-injectors at $4,500, which was deemed to be expensive compared to Mylan’s $300 price tag for a two-pack of their branded generic version of the EpiPen. However, Kaléo has since lowered the cash price to $360, with most patients with commercial insurance paying $0 out of pocket through the company’s AUVI-Q AffordAbility program.
While Kaléo has reportedly secured Health Canada approval to market the AUVI-Q in the country, the company has yet to launch their device.








Join or login to leave a comment
JOIN LOGIN