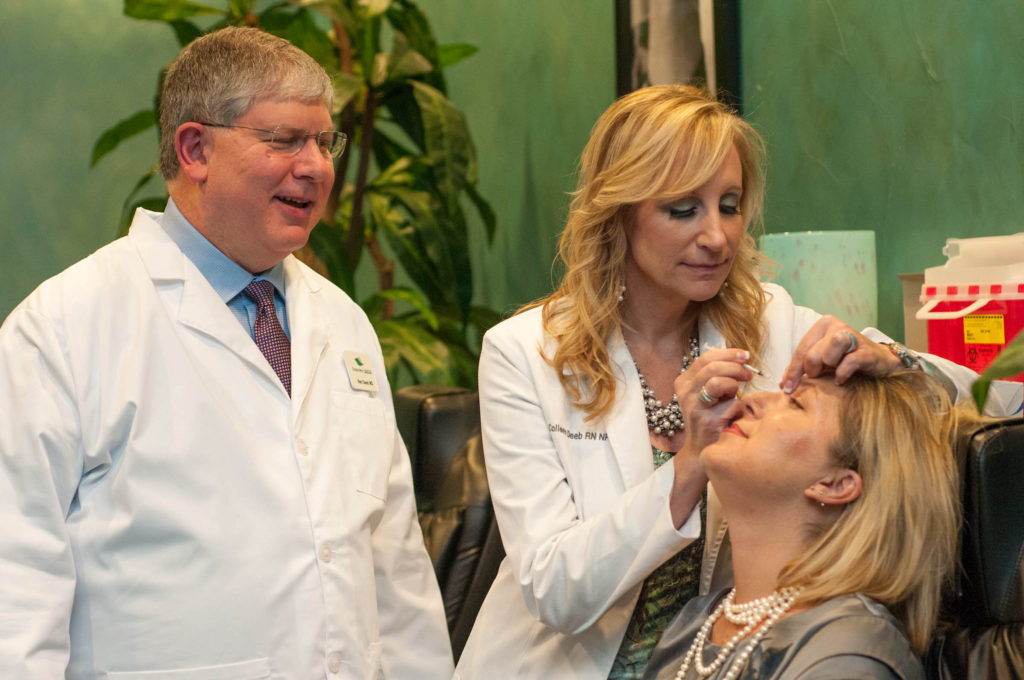
Since Mylan and Revance Therapeutics announced they were developing a biosimilar version of the blockbuster cosmetic drug Botox earlier this year, Allergan has launched a direct-to-consumer (DTC) ad campaign aimed at building brand loyalty for Botox. The primary message of the ads – one of which is aimed at women, while the other is targeted towards men – is to get patients to ask for Botox by name to avoid getting a likely less expensive biosimilar, if and when it’s approved.
The female-targeted ad features close-ups of the facial features of two women – a photographer and a dancer – while they’re going through their daily lives. The DTC commercial highlights the fact that Botox is the only cosmetic approved by the US Food and Drug Administration (FDA) for temporary improvement in moderate to severe crow’s feet, forehead lines and frown lines.
“Face the world as a face to be reckoned with,” says the female narrator in the first Botox ad. “There’s only one Botox Cosmetic – ask for it by name.”
The second commercial features the same upbeat background music as the first, but highlights three men, each of which focuses on the “details” in their lives. A man wearing a suit is seen straightening his tie and adjusting his cufflinks, while another man is pictured using a straightedge on an architectural drawing. The final actor is shown jogging through a wooded area and stopping to tie his shoelace and look at his fitness tracking watch.
“It’s the details that make the difference,” says the male narrator in the second Botox ad. “Ask your doctor about Botox Cosmetic by name.”
Mylan and Revance Thrapeutics’ Botox biosimilar RT002 has already completed a set of two Phase III clinical trials. The collaborators plan on pursuing approval for multiple indications of the Botox biosimilar with the aim of commercializing the injectable in both the US and globally.
But according to some analysts, the fact that Allergan offers bulk discount deals to clinics who use an assortment of the company’s cosmetic drugs – including Juvéderm and Latisse – could prevent a Botox biosimilar from gaining significant market share. It’s also unclear whether Mylan and Revance will look to secure FDA approval for all indications of Botox, however previously-approved biosimilars in other therapeutic areas have gained approval for many – if not all – of the indications of their reference product.
Last year, Allergan received approval to market Botox for the treatment of forehead lines. Most recently, a study conducted by researchers at Houston Methodist Hospital found that Botox injections could treat patients who grind their teeth at night, a condition known as bruxism.
Currently, Allergan’s Botox has secured nine indications from the FDA including in the treatment of bladder dysfunction and chronic migraine. Allergan reported sales of Botox to the tune of $817 million in the first quarter of 2018, representing a 14.5 percent increase over the same period in 2017.



Join or login to leave a comment
JOIN LOGIN