Johnson & Johnson (J&J) MedTech recently received US Food and Drug Administration (FDA) approval for its VARIPULSE pulsed field ablation (PFA) platform, making it the first PFA system in the US to be fully integrated with the CARTO 3 electro-anatomical mapping system for treating paroxysmal atrial fibrillation (AFib). This approval paves the way for a more efficient, precise treatment approach for AFib, an irregular heart rhythm that affects millions globally.
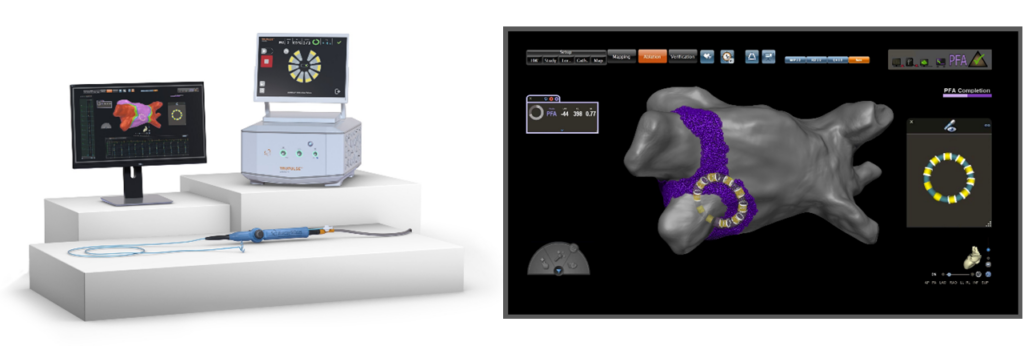
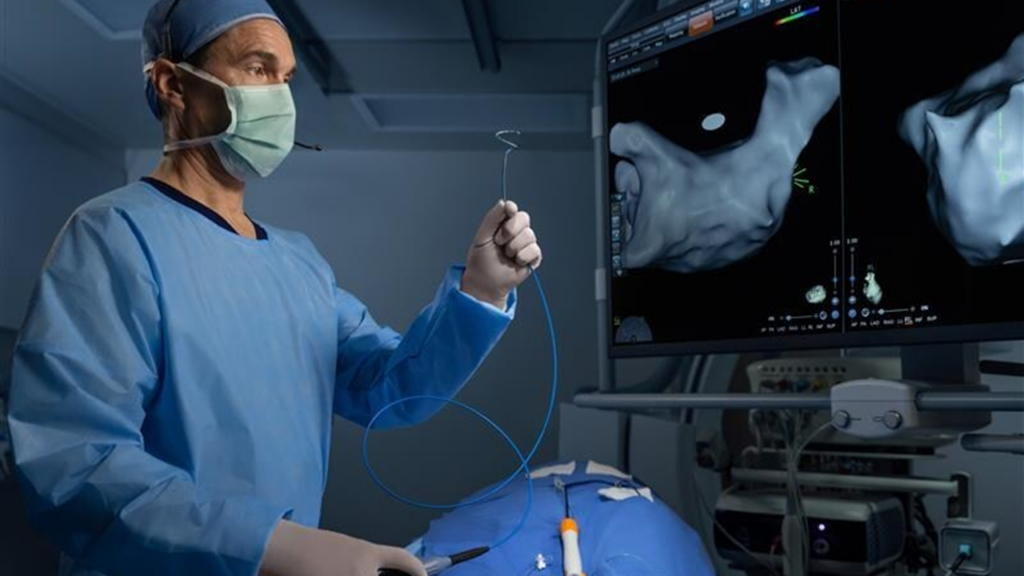
The VARIPULSE platform combines PFA technology with advanced mapping capabilities. PFA, a non-thermal ablation method, uses pulsed electric fields to selectively target heart tissue without affecting surrounding structures, unlike traditional heat-based methods. By integrating with the CARTO 3 system, electrophysiologists — specialists in treating abnormal heart rhythms — can visualize catheter positioning and tissue proximity in real-time, enhancing procedural accuracy and potentially reducing treatment duration.
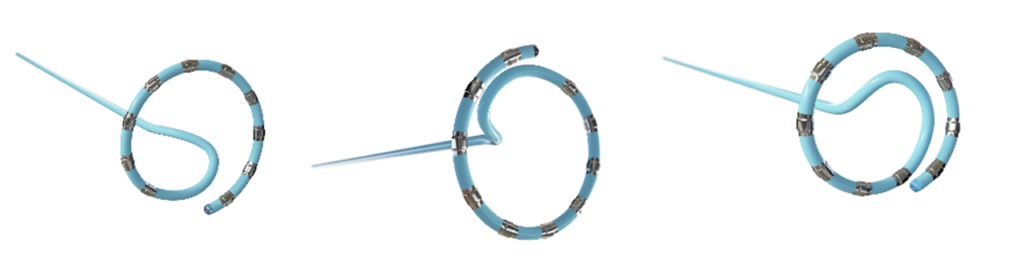
The platform’s flexible catheter loop, available in multiple sizes, allows for a customized fit based on each patient’s anatomy. The CARTO 3 system also supports lesion tagging, which “marks” ablated areas on the heart’s map, ensuring precise energy delivery without overlap — critical for long-term treatment success. Additionally, the platform minimizes the need for fluoroscopy (X-ray imaging), reducing radiation exposure for patients and providers.
The approval is backed by the admIRE study, a multi-center clinical trial across 30 US healthcare facilities involving 291 patients. The study demonstrated a 100 percent acute procedural success rate and 85 percent achieving sustained heart rhythm normalization over 12 months. Among these procedures, 25 percent were completed without the need for fluoroscopy, reflecting the integration benefits with CARTO 3.
XTALKS WEBINAR: How EHR Data Can be Used to Advance Guideline-directed Care
Live and On-Demand: Thursday, December 5, 2024, at 12pm EST (9am PST)
Register for this free webinar to learn how complete electronic health record (EHR) data are advancing medical device performance and improving patient outcomes.
AFib remains the most common type of cardiac arrhythmia, affecting over eight million Americans, with one-third of cases going undiagnosed until complications arise. This highlights the need for advanced technologies like VARIPULSE to help address treatment gaps and improve patient outcomes.
The company is expanding its PFA portfolio, including the investigational Dual Energy Thermocool Smartouch SF Catheter and Omnypulse Catheter, designed to further enhance precision and flexibility for electrophysiologists.
The VARIPULSE platform will be taking on Medtronic’s newly approved Affera Mapping and Ablation system, both aimed at advancing PFA for arrhythmia treatment. While VARIPULSE focuses on precise PFA treatment for paroxysmal AFib with its single-energy approach and integration with the CARTO 3 mapping system, Medtronic’s Affera system offers a dual-energy option — PFA and radiofrequency (RF ) — for treating more complex cases like persistent AFib. This positioning brings a competitive spin to the AFib treatment space, with both platforms aiming to boost procedural efficiency and treatment outcomes.
Another major player, Abbott, is developing its Volt PFA system, which pairs with its EnSite X mapping platform and recently completed enrollment in a large trial aimed at advancing its investigational PFA catheter for clinical use.
This rising interest highlights the broader industry shift toward advanced mapping and PFA technologies to support AFib treatment across diverse patient needs.









Join or login to leave a comment
JOIN LOGIN