Inhalation exposure systems for preclinical research have evolved since their introduction in the early 1900s to meet the needs of many different research applications. These systems vary significantly depending on the type of research being conducted, which can include: Inhalation Toxicology, Biodefense Aerobiology, Pharmaceutical, Discovery and Drug Development, Basic Respiratory Physiology, and Environmental Toxicology and Exposure studies. With each of these research disciplines, the scientific approach will differ, as will the complexity of the study design and the desired endpoints. As a result, the type of equipment required will also differ. With each inhalation delivery method and application, a host of benefits and potential disadvantages follow.
Delivering aerosol compounds for preclinical inhalation research has been historically challenging. Even today, many inhalation exposure systems require large amounts of API (active pharmaceutical ingredient) and invasive techniques that necessitate anesthesia and intratracheal administration. Such techniques have potential disadvantages because they are not representative of methods used in clinical settings. The use of mass dosing chambers for delivery of an inhaled substance eliminates the issue of anesthesia and allows the animal to be unrestrained and roam freely. However, this approach may result in excess use of API and be an inefficient method to deliver compound to the animal’s lungs.
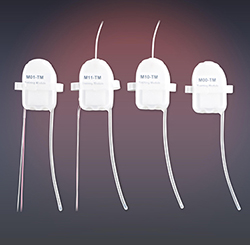
Scientists from Merck & Co. pharmaceuticals determined that there was a need to develop a superior system for the delivery of liquid and dry powder aerosols. With input from Merck, Buxco Research Systems (which was acquired by Data Sciences International [DSI] in 2014) developed and validated a 14-port inhalation tower. The tower, with its all-in-one controller, patented AllayTM restraint collar for rodents, and nose-only plethysmograph to deliver small amounts of API continuously to conscious rats, gives the researcher peace of mind that an inhaled substance will be delivered to all animals in a uniform and reproducible manner.
This webinar will include a discussion about inhalation exposure systems, specifically:
- The variety of inhalation exposure applications currently being used in research, along with their advantages and disadvantages
- Identifying the need for better inhalation exposure tools
- Introduction of DSI’s 14-Port Inhalation Tower and All-in-One Tower Controller
- Scientific perspective of 14-Port Inhalation Tower
The 14-Port Inhalation Tower now allows Merck to:
- Minimize systemic exposure from fur or skin contamination utilizing nose-only delivery
- Deliver compound to conscious animals and eliminate complications associated with anesthesia
- Decrease API amounts required, therefore minimizing the significant investment associated with compound scale-up, through controlled delivery
- Utilize the flexible design of the inhalation tower that allows the use of existing/conventional methods for restraint (push bar or barrier placed at the rear of the animal) or the use of the Allay restraint method
- Obtain real-time measurement of changes in respiratory physiology during compound delivery in the same experiment when the 14-Port Inhalation Tower and the Allay restraint collar are used with a nose-only plethysmograph. This is a significant advancement for inhalation delivery because it uses the real-time minute ventilation measurement for delivered dose (DD) calculations, circumventing DD calculations based on estimated minute ventilation (RMV) and body weight
The 14-Port Inhalation Tower used with the Allay restraint collar and nose-only plethysmograph system yields reproducible, high-quality ventilation data for the duration of compound delivery and over a large range of flows. Merck has evaluated the 14-Port Inhalation Tower in conjunction with HD-S21 dual channel transmitters to evaluate pulmonary and systemic hemodynamics post-delivery.
Speakers

Michael Girand, Principal Applications Engineer, Data Sciences International (DSI)
Mike Girand is a Principal Applications Engineer at Data Sciences International (DSI). Since 2007, responsibilities at DSI have also included sales, technical and application support, and product management. Prior to joining DSI, Mike was a Research Scientist and Study Director at Warner-Lambert/Parke-Davis and Pfizer in Ann Arbor, MI for 12 years. While there, he had the opportunity to work in a variety of departments including Cardiovascular Pharmacology, General Toxicology, and Safety Pharmacology. In 1992, Mike received his undergraduate degree in Zoology at Michigan State University and in 2005 completed his MBA at the University of Phoenix. His areas of interest include respiratory and cardiovascular hardwired application

Aileen House, Sr. Scientist, In Vivo Pharmacology – Cardiovascular Department, Merck & Co Pharmaceuticals
Aileen House started her career at Schering-Plough in the Allergy and Inflammation Department. The company underwent several changes and today she is a Sr. Scientist in the In Vivo Pharmacology-Cardiovascular Department at Merck. She began working in Respiratory Disease in 1987, and has worked on a variety of endpoints and species including rats, mice, guinea pigs, dogs and monkeys looking at neuronal mechanisms controlling pulmonary mechanical function, LT antagonists, H3 agonists, sGC compounds, dissociated steroids, just to name a few. Many of her early projects were in guinea pig models of lung dysfunction, but she soon moved into work measuring allergic bronchoconstriction and airway hyperreactivity in non-human primates to investigate the effect of an anti-IL-5 mono-clonal antibody. From there she developed an allergic Brown Norway rat model and expanded into work on NK antagonists, PDE4 antagonists and CRTH2 antagonists. Her expertise with large animals was expanded when she performed studies in dogs to investigate allergic bronchoconstriction, basic respiratory function, and cough. Aileen is currently in the In Vivo Pharmacology Department at Merck. Ms. House performs studies to develop novel ways to deliver Active Pharmaceutical Ingredient (API) to rats and dogs.
Who Should Attend?
This webinar is intended for anyone working in preclinical biomedical research who has an interest in administering inhaled substances and monitoring respiratory endpoints in research subjects. Attendees may include research scientists and technicians, veterinarians, study directors, and biomedical engineers. The audience will learn about various respiratory inhalation applications and the new possibilities DSI’s 14-port inhalation tower enables for an accurate, reliable inhalation research solution.
Xtalks Partner
DSI
DSI is a pioneering biomedical research company focused on preclinical systems physiology and pharmacology. The recognized global leader in physiologic monitoring, DSI offers telemetry, instrumentation, software and services that facilitate accelerated, well-informed, drug therapy and development decisions.
DSI serves many industries including: Pharmaceuticals, Academia, Contract Research Organizations, Biological and Chemical defense, the Medical Device Industry, Government, and Biotechnology companies. We offer solutions that are tailored specifically to meet the unique research needs of our customers.
Media Partner
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account