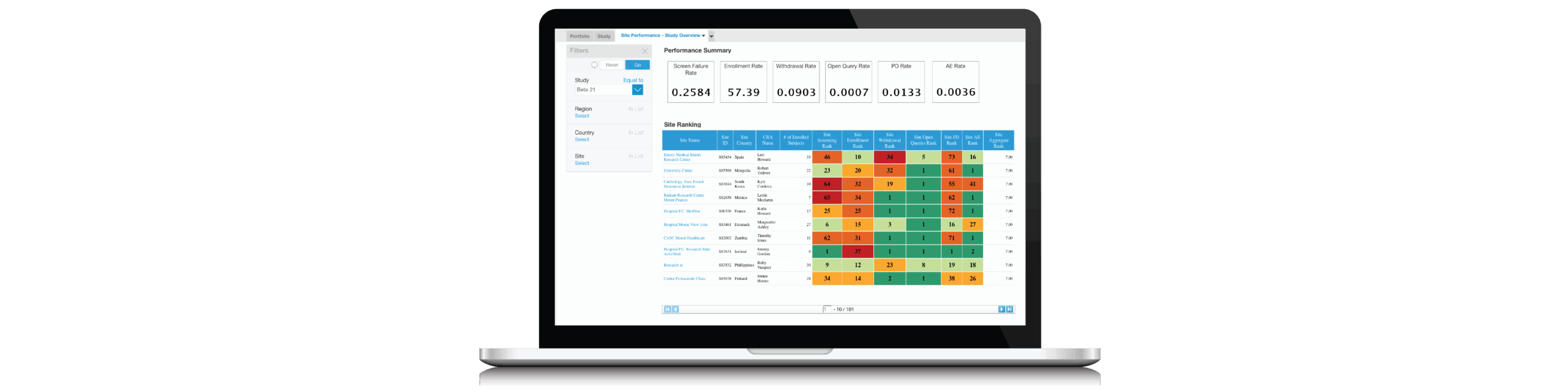
Thanks to ICH E6(R2), the guideline for Good Clinical Practice, and other regulatory guidance, sponsors and CROs are motivated to apply risk-based monitoring (RBM) strategies to their clinical trials. The fact that the non-profit, TransCelerate, recently updated its Key Risk Indicators (KRI) library is an important indicator that RBM approaches are gaining traction.
In most cases, however, RBM approaches fail to provide workflows that take into account how human beings like to get information. Dashboards alone don’t cut it.
Using examples from eCommerce and online stock trading platforms, this thought-provoking webinar will explain the value of event-driven information delivery and how it can be applied to the clinical trial industry. By focusing on “events of interest,” participants will gain the ability to proactively identify risk at the portfolio, study, region, investigator and site levels.
Through a real-world pharmaceutical success story, participants will learn how to implement an event-driven RBM system that can:
- Aggregate data from different systems automatically
- Generate performance alerts at predetermined thresholds
- Facilitate collaboration through a fully auditable task management system
Speaker

Chandi Kodthiwada, Senior Product Manager, Comprehend
As a product leader and a strategist for more than a decade, Chandi has helped more than 60 pharma and biotech companies harness the power of data to better manage clinical trial complexity. Before joining the Comprehend team, Chandi helped build clinical and operational analytics platforms for a number of pharmaceutical companies, CROs and technology vendors. As Comprehend’s senior product manager, he is responsible for enhancing the company’s current products and developing new innovations to support their customers.
Chandi holds a Master’s of Science in Computer Science from the New Jersey Institute of Technology.
Who Should Attend?
This webinar will appeal to:
CROs, Clinical Operations and Data Management Professionals working in:
- Clinical Trial/Clinical Study Management
- Clinical Data/Informatics/IT
- Clinical Outsourcing
- Clinical Project Managers
- Clinical Project Directors
- Director of Project Delivery
Clinical Research, Technology and Business Professionals working in:
- Biometrics/Biostatistics
- Business Technology/Applications/Solutions
- Business Analyst
- CTO
- Project Management
What You Will Learn
In this free webinar, learn how to implement an event-driven RBM system that can:
- Aggregate data from different systems automatically
- Generate performance alerts at predetermined thresholds
- Facilitate collaboration through a fully auditable task management system
Xtalks Partner
Comprehend
Comprehend provides a suite of cloud applications and consulting services that dramatically improve the clinical trial process. Our solutions deliver actionable risk and performance insights across studies, systems, sites, and vendors. By using elements of our Clinical Intelligence Platform to unify, monitor, and analyze data across all sources, sponsors and CROs alike are able to reduce risk, achieve milestones on time, and stay within budget. As a trusted partner, Comprehend helps speed the time to quality results.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account