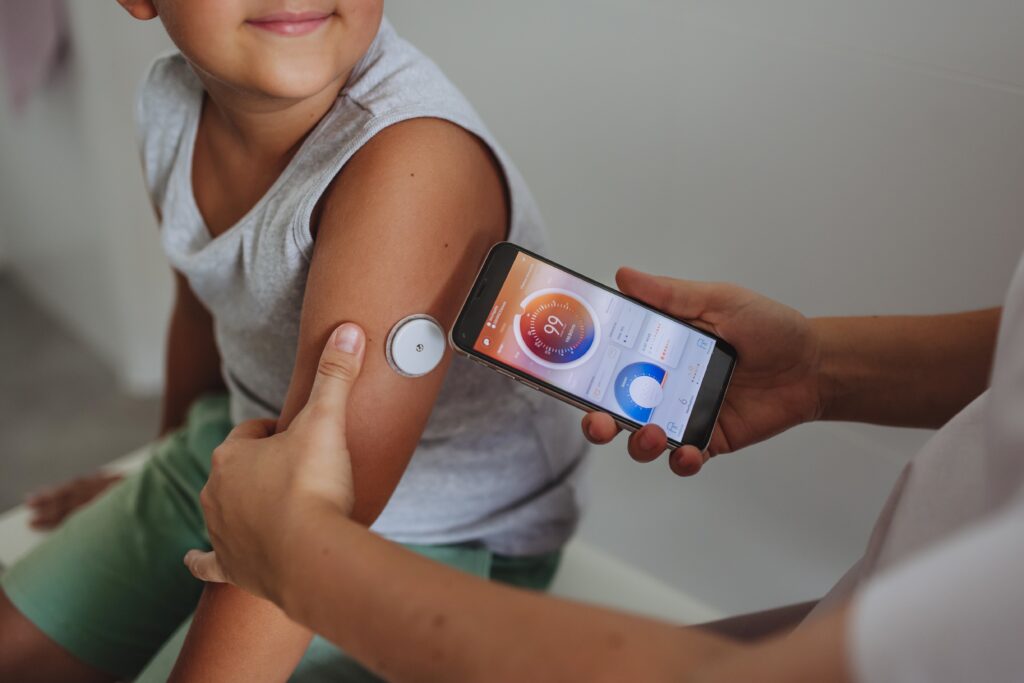
Abbott and Medtronic have struck a global partnership to make diabetes management easier for people who need regular insulin injections. The partnership will involve pairing Abbott’s leading FreeStyle Libre technology for continuous glucose monitoring (CGM) with Medtronic’s automated insulin delivery (AID) and smart insulin pen systems.
Abbott’s CGM sensors will work with Medtronic’s AID algorithms to automatically adjust insulin levels based on real-time blood sugar readings. This could make life much simpler for people with diabetes, especially those with Type 1 and Type 2 diabetes, who need to monitor their blood sugar frequently and take multiple insulin injections each day. The technology is expected to help keep blood sugar levels within a healthy range, reducing the need for constant decision-making.
XTALKS WEBINAR: New Era of Obesity Drug Development
Live and On-Demand: Thursday, September 19, 2024, at 10am EDT (3pm BST/UK)
Register for this webinar today to understand the latest advancements related to obesity pharmacotherapy.
Abbott will develop the sensors designed specifically for Medtronic devices but they will be sold by Medtronic. The partnership is part of Abbott’s broader strategy to offer more options and flexibility to people living with diabetes by partnering with other major companies in the field.
In a press release, Jared Watkin, executive vice president of Abbott’s diabetes care business emphasized that the collaboration is intended to ease routine diabetes care and allow people to focus more on living their lives. Que Dallara, executive vice president and president of Medtronic Diabetes, added that the integration will help make advanced insulin delivery systems more accessible.
Related: Dexcom Unveils Stelo, the First OTC Glucose Biosensor, Coming Summer 2024
In parallel with this partnership, Medtronic recently received US Food and Drug Administration (FDA) approval for its Simplera CGM, the company’s first all-in-one disposable CGM that is half the size of previous models. Its discreet design enables easy insertion without the need the need for overtape, offering greater ease and comfort for daily users. Simplera is designed to be used with Medtronic’s InPen smart insulin pens and the MiniMed 780G system, which has already received positive feedback in Europe.
The global glucose monitoring devices market is projected to reach $38.9 billion by 2033. The rise in diabetes prevalence, coupled with increased awareness and education, are driving the expansion.
Despite being hit with some recalls, Abbott continues to be a key player in this segment. The CGM market has seen a steady wave of new devices from Abbott, including recent over-the-counter systems Lingo and Libre Rio, which are biowearables designed to help provide personalized well-being insights and non-insulin based lifestyle management recommendations, respectively.











Join or login to leave a comment
JOIN LOGIN