AstraZeneca has scored a regulatory win for its hairy cell leukemia drug Lumoxiti (moxetumomab pasudotox-tdfk), representing the first new treatment option for patients with relapsed or refractory forms of the disease in more than 20 years. The FDA approved the intravenous drug as a third-line therapy for adult patients who have previously been treated with a purine nucleoside analog or other systemic therapy.
“Today’s FDA approval of Lumoxiti represents a significant milestone for people living with hairy cell leukemia, a rare blood cancer that can result in serious and life-threatening conditions,” said Dave Fredrickson, Executive Vice-President, Global Head Oncology Business Unit for AstraZeneca. “For patients, this approval provides the first FDA-approved medicine for this condition in more than 20 years.”
The approval was based on data from the 80-patient open-label ‘1053’ Phase III trial which found that 75 percent of patients treated with Lumoxiti achieved an overall response. What’s more, 30 percent of patients with hairy cell leukemia had a durable complete response after being treated with Lumoxiti.
“While many patients with hairy cell leukemia experience a remission with current treatments, 30 percent to 40 percent will relapse five to ten years after their first treatment,” said Dr. Robert J. Kreitman, Senior Investigator, Head of Clinical Immunotherapy Section, Laboratory of Molecular Biology, Center for Cancer Research, National Cancer Institute. “With subsequent treatments, durations of response diminish and toxicities accumulate, and few approved treatment options exist. Moxetumomab pasudotox represents a promising non-chemotherapeutic agent for [hairy cell leukemia], addressing an unmet medical need for physicians and their patients.”
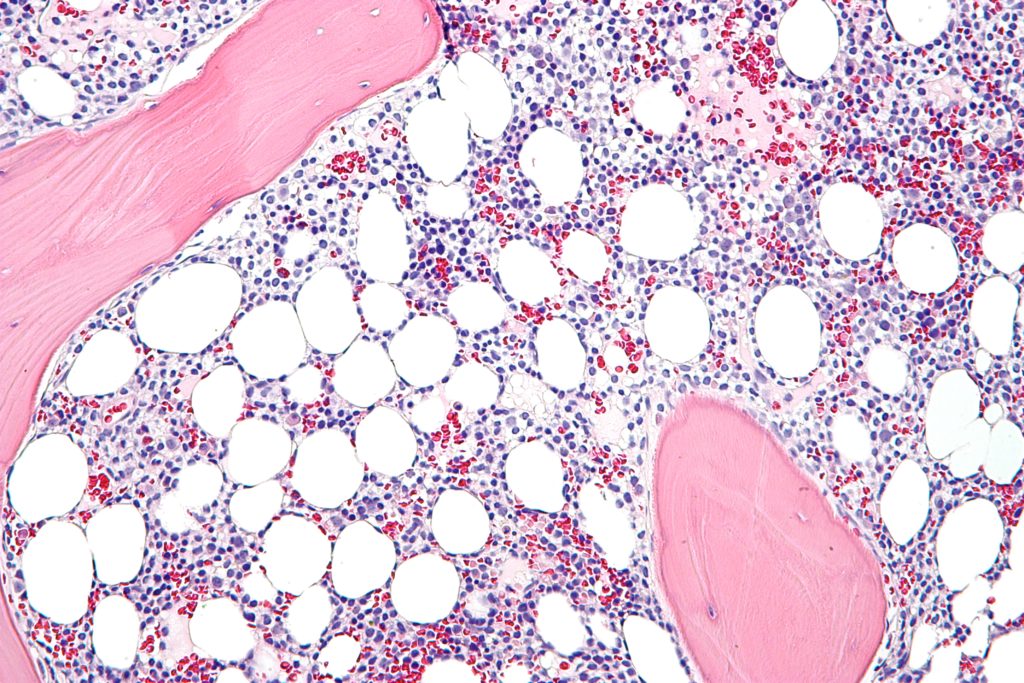
Each year, about 1,000 individuals in the US are diagnosed with hairy cell leukemia, a rare form of the blood cancer characterized by the production of abnormal B cell lymphocytes by the bone marrow. Up to 40 percent of patients who respond to treatment will relapse within 10 years, which means there is a need for more second- and third-line therapies.
Lumoxiti meets that unmet medical need by acting as a CD22-directed cytotoxin which binds to the leukemia cells. The second component of lumoxiti is a truncated bacterial toxin which triggers cell death by preventing protein synthesis. The drug is a first-in-class treatment which was previously granted Orphan Drug designation by the FDA.
“Lumoxiti fills an unmet need for patients with hairy cell leukemia whose disease has progressed after trying other FDA-approved therapies,” said Dr. Richard Pazdur, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “This therapy is the result of important research conducted by the National Cancer Institute that led to the development and clinical trials of this new type of treatment for patients with this rare blood cancer.”








Join or login to leave a comment
JOIN LOGIN