Imagine a family of five affected by the same disease that prevents their heart from pumping normally. However, each member is affected differently: the children have a severe form, the father has a mild form and the mother is asymptomatic. What could explain this phenomenon?
According to research published in Science, scientists traced the origins of left ventricular noncompaction to three genetic mutations whose interactions result in the different severities of disease.
“Given the severity of the disease in the children and the fact that one of the parents had an asymptomatic form, we suspected that the condition in the children was caused by a combination of the mother and the father’s genes,” said Dr. Deepak Srivastava, a cardiologist at UCSF Benioff Children’s Hospitals and senior author of the paper.
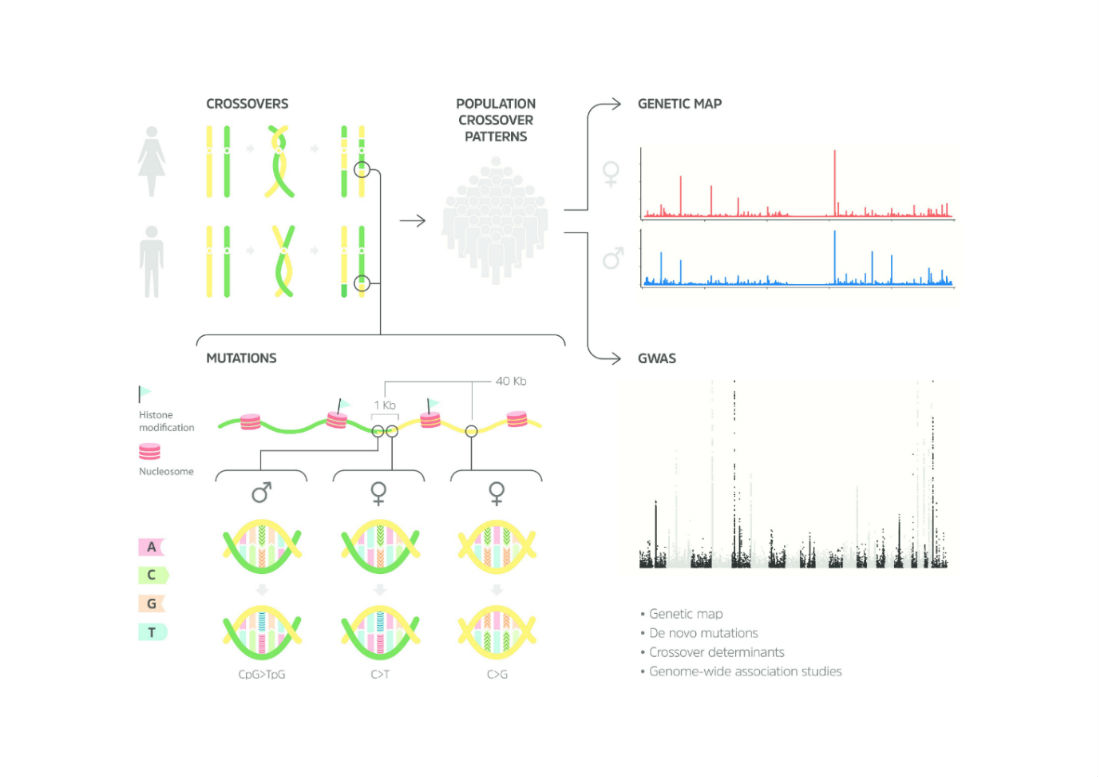
Dr. Srivastava and his team from Gladstone Institutes and the University of California, San Francisco used advanced genetic techniques to assess the genomes of all five family members. Their analysis revealed two mutations in the father’s genes that resulted in his milder disease and one mutation in the mother’s genes that did not affect her heart at all. But when the combination of mutations was inherited together, the children had a severe form of the disease.
“Our findings suggest that the gene inherited from the mother exacerbated the problem caused by the father’s genes, resulting in a much more severe form of heart disease in the children,” said Dr. Casey Gifford, a staff scientist at Gladstone Institutes and first author of the paper.
To validate their findings, the team derived heart cells from stem cells taken from each family member. When the heart cells were mature, they displayed the same abnormalities as seen in the person from which they were derived from. In other words, heart cells derived from the children’s stem cells showed signs of disease.
It’s not uncommon to find multiple genetic mutations at work in human disease. Consider the various forms of breast cancer associated with the HER2 gene. Between two women with hormone-receptor-negative breast cancers, one could have a rarer, more aggressive form (triple negative breast cancer) while the other has a more common form (HER2-positive breast cancer). The type and severity of these breast cancers depend on the absence (triple negative) or presence (HER2-positive) of the HER2 protein.
Similarly, genetic variations partly explain the diversity in our appearances. Within a family, for example, the combination of genes between a mother and father can result in two children with different shades (hazel, caramel or chestnut) of the same eye color (brown).
As the tools to study genetics become more refined, our capacity to treat complex human pathologies are likely to improve. The same gene editing technology used to reveal the cause of congenital heart disease has been used to correct mutations associated with Duchenne Muscular Dystrophy (DMD). Scientists have also found ways to “grow” functional organoids outside the body to potentially repair or replace damaged ones. With continued research, people living with congenital heart disease and other complex genetic disorders may have access to more effective therapies.









Join or login to leave a comment
JOIN LOGIN