This week, LEO Pharma announced the publication of pivotal Phase III clinical trial results in The Lancet for its topical delgocitinib cream to treat severe chronic hand eczema (CHE).
Results from the trial showed delgocitinib to have superior efficacy and safety over oral alitretinoin, the current standard of care for CHE.
The DELTA FORCE trial is the first head-to-head Phase III study comparing a topical treatment with a systemic therapy for CHE.
Conducted across 102 sites in Europe and Canada, the 24-week, randomized, assessor-blinded trial enrolled 513 adult participants with severe CHE. Patients were assigned to receive either delgocitinib cream (20 mg/g) applied twice daily or oral alitretinoin (30 mg) taken once daily.
Delgocitinib cream met the primary endpoint in the trial, demonstrating a significantly greater reduction in Hand Eczema Severity Index (HECSI) scores at Week 12 compared to alitretinoin.
It also met all key secondary outcome endpoints, including outperforming alitretinoin in achieving treatment success based on the Investigator’s Global Assessment for CHE (IGA-CHE). It also improved health-related quality of life, as measured by the Dermatology Life Quality Index (DLQI).
Alitretinoin is currently the only approved treatment in Canada for severe CHE in patients who do not respond to topical corticosteroids.
XTALKS WEBINAR: Beyond Spreadsheets: How Real-Time Dashboards are Transforming Transparency in Clinical Research
Live and On-Demand: Friday, June 6, 2025, at 3pm EDT (12pm PDT)
Register for this free webinar to learn how unified systems and real-time dashboards can streamline clinical operations. Discover how they can reduce data silos and enhance trial performance.
In both Canada and the US, delgocitinib cream remains under regulatory review and has not yet been approved by Health Canada or the FDA.
In September, LEO Pharma announced that the FDA had accepted its application for the topical cream to treat adults with CHE. A decision is anticipated in the second half of this year. If approved, it would become the first therapy specifically authorized for CHE in the US.
Delgocitinib cream, marketed as Anzupgo in the European Union (EU), UK, Switzerland and the United Arab Emirates (UAE), is approved for treating moderate to severe CHE in adults unresponsive to topical corticosteroids.
In the trial, patients treated with delgocitinib reported fewer treatment-emergent adverse events (49%) compared to those on alitretinoin (76%). Common adverse events for alitretinoin included headaches (32%) and nausea (6%), whereas delgocitinib had notably lower incidences.
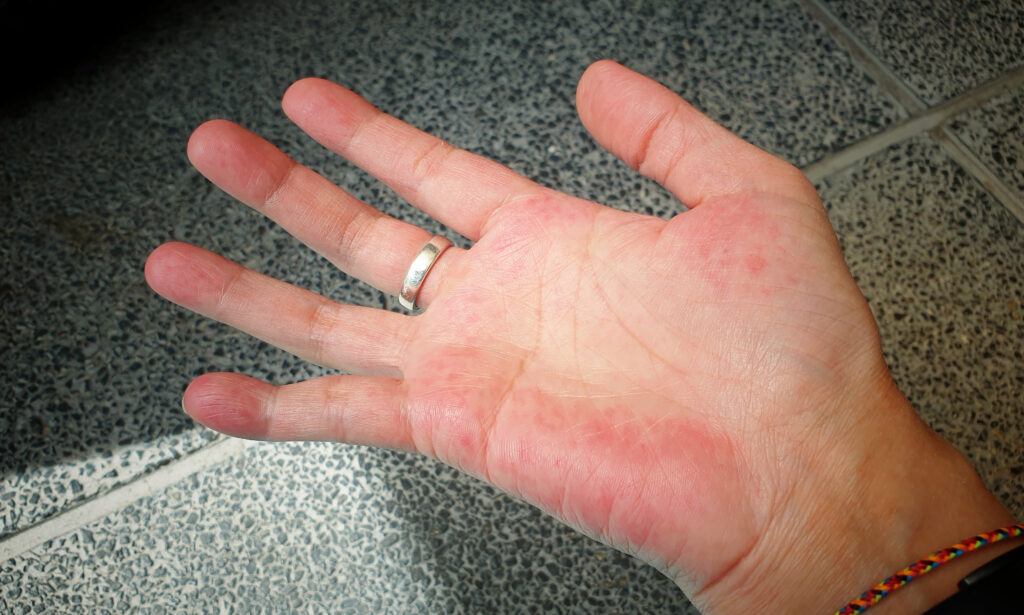
CHE is characterized by persistent or recurrent inflammation of the hands. Symptoms include redness, scaling and fissures. With a prevalence of approximately 4.7%, CHE significantly impacts patients’ quality of life, affecting daily activities and occupational functioning.
Delgocitinib cream is a topical pan-Janus kinase (JAK) inhibitor. It inhibits the activation of JAK-STAT signaling, which plays a key role in the pathogenesis of CHE. Its pathophysiology includes skin barrier dysfunction, inflammation of the skin and alterations of the skin microbiome.
Robert Spurr, EVP of LEO Pharma North America, remarked, “The discussion of delgocitinib and the presentation of DELTA FORCE data in The Lancet is an exciting moment for the LEO Pharma team. We are proud to contribute to the overall understanding of moderate to severe CHE, helping uncover potentially new treatment options for those living with skin conditions like CHE.”
Dr. Charles Lynde, chief medical director at The Lynde Institute for Dermatology and Associate Professor at the University of Toronto, emphasized the significance of the findings: “We know that CHE is an under-researched condition. We hope that discussions of the DELTA FORCE program, like that published in The Lancet, will continue to raise awareness of the disease, inspire new research and ultimately help improve the quality of life of those living with CHE.”
Related: Orismilast Closer to Phase III as a Promising Oral Treatment for Atopic Dermatitis
Last month, Leo Pharma shared data from an Ipsos survey it commissioned, which revealed that dermatology clinicians feel there is a lack of awareness and understanding of CHE.
According to the first phase of the survey, 65% of dermatology providers said there is a lack of education and understanding of CHE as a condition distinct from atopic dermatitis (AD).
Additionally, more than 90% of the surveyed providers reported that their patients with moderate to severe CHE experience significant physical and emotional impacts from the disease.
Moreover, 92% of providers said CHE limits their patients’ ability to perform daily activities, with tasks like typing at a computer. The condition also impacts workplace performance, with 86% reporting that CHE has caused work-related struggles for their patients.
The CHE and broader eczema treatment markets are becoming increasingly competitive, with several major pharma companies and emerging biotech firms advancing new therapies.
While LEO Pharma is leading in the CHE space with delgocitinib, Asana BioSciences is developing ASN-002, a dual JAK/SYK inhibitor for CHE.
In the broader eczema market, Sanofi and Regeneron dominate with their blockbuster IL-4/IL-13 inhibitor Dupixent (dupilumab). Eli Lilly recently launched Ebglyss (lebrikizumab), an IL-13 inhibitor approved in 2024. Pfizer’s Cibinqo (abrocitinib) and AbbVie’s Rinvoq (upadacitinib), both oral JAK inhibitors, also offer alternatives for moderate-to-severe AD.
Arcutis Biotherapeutics’s eczema cream Zoryve (roflumilast), a topical PDE4 inhibitor, has been on the market since 2022, indicated for the treatment of plaque psoriasis.
The global eczema therapeutics market is projected to reach $37.1 billion by 2034.
If you want to have your organization featured on Xtalks, please email Ayesha Rashid at: [email protected]








Join or login to leave a comment
JOIN LOGIN