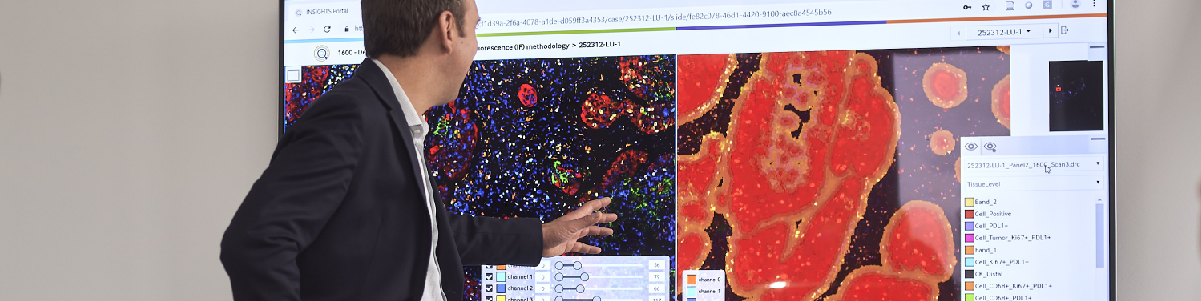
To the untrained eye, the picture below might look like blobs of pink and purple circles. But to a cancer pathologist, this is a digitized histology slide showing numerous tumors throughout bone.
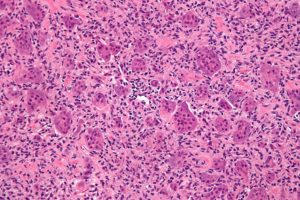
Not all cancers look like this textbook example, however. Among recent advances in cancer research is the use of artificial intelligence (AI) to assist with cancer diagnosis, but until recently, these technologies have not been approved for use by regulatory authorities.
Early March, computational pathology company Paige.AI received breakthrough device designation from the US Food and Drug Administration (FDA) for an artificial intelligence (AI)-driven cancer diagnostics platform.
“We are honored to have been granted Breakthrough designation by the FDA, which underscores the groundbreaking nature of our technology as the leading clinical-grade AI in computational pathology to combine vast amounts of high-quality data with unique deep learning architectures in service of delivering better patient care,” said Dr. Thomas Fuchs, Co-Founder of Paige.AI in a press release.
The algorithm has been trained with thousands of digitized pathology slides and thus has the capacity to diagnose new cases. The digitized slides come from Memorial Sloan Kettering (MSK), a leading cancer research center based in New York.
In an agreement with Paige.AI, researchers from MSK provide images of digitized pathology slides to the AI company, amassing more than one million slides to date. Digitizing slides has gained popularity in recent years as images can be shared and reviewed by multiple experts to help make accurate diagnoses.
For these AI-driven platforms to function, large quantities of data are needed. MSK is funding the digitization of four million additional slides to create the world’s largest digitized pathology set. With FDA Breakthrough Device Designation, Paige.AI’s platform might be eligible for accelerated regulatory approval.
Disease diagnostics is only the tip of the iceberg for AI applications in healthcare. Big pharma has dappled into AI technologies through drug discovery and development, medication adherence, predicting patient outcomes, pharmacovigilance and optimizing clinical trial operations. In January, Microsoft and Walgreens joined forces to tackle these different areas, with the hope of changing the patient experience.
While AI promises to ‘revolutionize healthcare’, there are several safety and quality concerns that must be addressed. In a review published in BMJ Quality & Safety, the authors suggest that AI and machine learning algorithms should be trained on the real-world costs of false positives or false negatives, not simply on arriving at the correct diagnosis. For example, the cost of misdiagnosing a woman of being cancer-free when she does have cancer (false negative) might be greater than the cost of the opposite scenario. In this case, the woman would be missing out on treatments and physician monitoring that could save her life.
It’s possible that AI platforms can correct human errors when examining a questionable cancer pathology slide, but even such advanced technologies can make mistakes. For now, AI and similar computer-aided diagnostic tools can provide guidance in disease diagnosis.
“Paige.AI is focused on providing artificial intelligence tools to pathologists that will enable them to become faster and more accurate in their diagnosis and treatment recommendations for the care of cancer patients,” said Dr. Leo Grady, Chief Executive Officer of Paige.AI.







Join or login to leave a comment
JOIN LOGIN