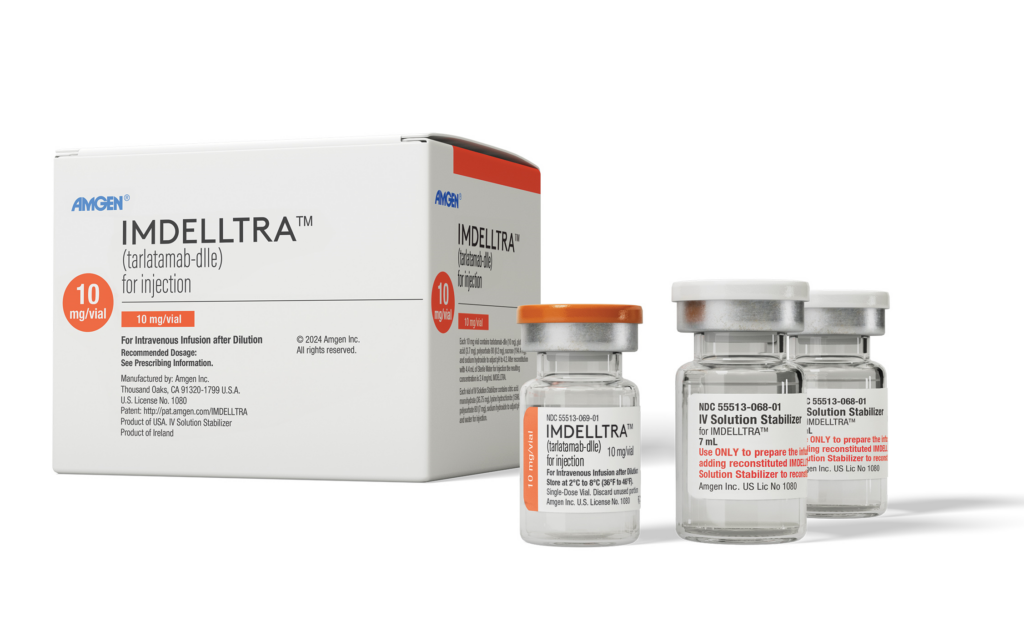
The US Food and Drug Administration (FDA) has approved Amgen’s Imdelltra (tarlatamab-dlle) for the treatment of extensive-stage small cell lung cancer (ES-SCLC) in patients who have experienced disease progression after platinum-based chemotherapy.
The approval is based on the overall response rate and duration of response observed in the DeLLphi-301 study, with continued approval depending on the demonstration of clinical benefit in a future confirmatory trial(s).
The new therapy targets delta-like ligand 3 (DLL3) on tumor cells, activating the patient’s T cells to attack and potentially destroy the cancer cells.
“Lung cancer is a complex and devastating disease, and less than three percent of patients with ES-SCLC live longer than five years,” said David P. Carbone, professor of internal medicine and director of the James Thoracic Oncology Center at the Ohio State University Medical Center, in a recent press release. Small cell lung cancer (SCLC) accounts for nearly 15 percent of lung cancer.
SCLC forms in the tissues of the lungs and spreads quickly to the other parts of the body, leading to early metastasis. Due to its aggressive nature, it has a poor prognosis and quickly develops resistance to chemotherapy. However, Imdelltra’s ability to impact cancer progression could provide a new therapeutic option for patients with this challenging disease.
Laurie Fenton Ambrose, co-founder, president and CEO of GO2 for Lung Cancer noted in the same press release, “After decades of minimal advancements in the SCLC treatment landscape, there is now an effective and innovative treatment option available.”
Jay Bradner, chief scientific officer at Amgen, added on this pivotal moment for patients battling ES-SCLC, “Imdelltra offers these patients who are in urgent need of new innovative therapies hope, and we’re proud to deliver this long-awaited effective treatment to them.”
XTALKS WEBINAR: AI-based Antibody Therapeutics Developability Assessment and Its Engineering
Live and On-Demand: Friday, June 21, 2024, at 10am EDT (4pm CEST/EU-Central)
Register for this webinar today to learn how AI can be used to improve developability screening of antibody therapeutics and optimization of antibody candidates.
A Targeted Bispecific Therapy and a First-In-Class Immunotherapy
Imdelltra was created with the help of Amgen’s BiTE (Bispecific T-cell Engager) technology which is designed to harness the body’s immune system to fight cancer. Tarlatamab is a BiTE molecule that is made of two parts, a part that attaches to T-cells and a part that attaches to cancer cells.
Imdelltra is a novel immunotherapy, where a bispecific antibody binds to both CD3 on T cells and DLL3 on tumor cells. This dual binding action brings T cells closer to cancer cells, prompting the T cells to attack and destroy the tumor cells. This method has proven to be a game-changer in oncology, providing new therapeutic options for hard-to-treat cancers like SCLC.
Tarlatamab Clinical Trial Insights
In the DeLLphi-301 Phase II study, tarlatamb was administered biweekly at a dose of 10 mg to patients with advanced SCLC who had progressed after two or more prior lines of therapy, aiming to activate their immune responses against the tumor.
The study’s effectiveness was measured by how many patients saw their cancer shrink and for how long. About 40 percent of patients saw significant shrinkage. On average, the reduction in cancer lasted about 9.7 months. The average survival time was 14.3 months, though final survival data are still being analyzed.
When looking specifically at patients based on their previous response to platinum-based therapy, 52 percent of those resistant to platinum-based chemotherapy (disease progression in less than 90 days of platinum-based therapy) saw shrinkage, while 31 percent of those sensitive but not resistant to platinum-based chemotherapy (disease progression after 90 days of platinum-based therapy) also saw shrinkage.
The most common adverse effects observed were cytokine release syndrome (CRS) in 55 percent of patients. Other side effects included fatigue (51 percent), pyrexia (36 percent), decreased appetite (34 percent), musculoskeletal pain and constipation (30 percent).
Boxed Warning with Imdelltra’s Approval
Imdelltra’s approval comes with a Boxed Warning for serious and life-threatening reactions, mainly CRS and neurologic toxicities, such as immune effector cell-associated neurotoxicity syndrome (ICANS). These serious adverse effects require careful monitoring and patient management.
The recommendation is to use the prescribed step-up dosing schedule while monitoring the occurrence and severity of CRS and neurologic toxicities. Should these severities worsen, Imdelltra is to be withheld immediately, and patients should seek hospitalization and further counsel.
Related: Imaging Drug Cytalux (pafolacianine) Approved for Detection of Lung Cancer During Surgery
The Road Ahead for Small Cell Lung Cancer Research
Those living with ES-SCLC can take heart in the monumental effort being put across many ongoing clinical trials. These trials are significant as they address an unmet medical need in ES-SCLC, known for its aggressive nature and limited treatment options after first-line therapy fails.
Through Amgen’s robust drug development program, tarlatamab continues to be explored through several ambitious clinical trials that aim to broaden its applications across different stages and types of cancer. For example, the DeLLphi-304 study is comparing tarlatamab monotherapy to standard chemotherapy in second-line SCLC treatment. In addition, the DeLLphi-305 trial is evaluating tarlatamab plus durvalumab versus durvalumab alone for first-line maintenance in ES-SCLC.
Daiichi Sankyo’s ongoing DS-7300 Phase II trial investigates the safety and efficacy of DS-7300, an antibody-drug conjugate (ADC; one of five ADCs in their oncology pipeline) targeting B7-H3, a protein often overexpressed in various cancers, including SCLC.
Another potential treatment for ES-SCLC is Puma Biotechnology’s alisertib which recently received Orphan Drug Designation by the FDA. Alisertib is being investigated in a Phase II study for its efficacy and safety. This selective, oral drug targets Aurora A kinase, a protein that plays a critical role in cell division, aiming to disrupt the proliferation of cancer cells.









Join or login to leave a comment
JOIN LOGIN