Update (August 30, 2018): Product Quest has expanded their product recall to include all nasal products as well as baby oral gels over concerns that these drugs could also be contaminated with Pseudomonas aeruginosa. While the manufacturer says the recall is being expanded “out of an abundance of caution,” these products were made at the same Florida production facility as the contaminated lots of CVS Health 12 Hour Sinus Relief Nasal Mist.
Originally published on August 9, 2018:
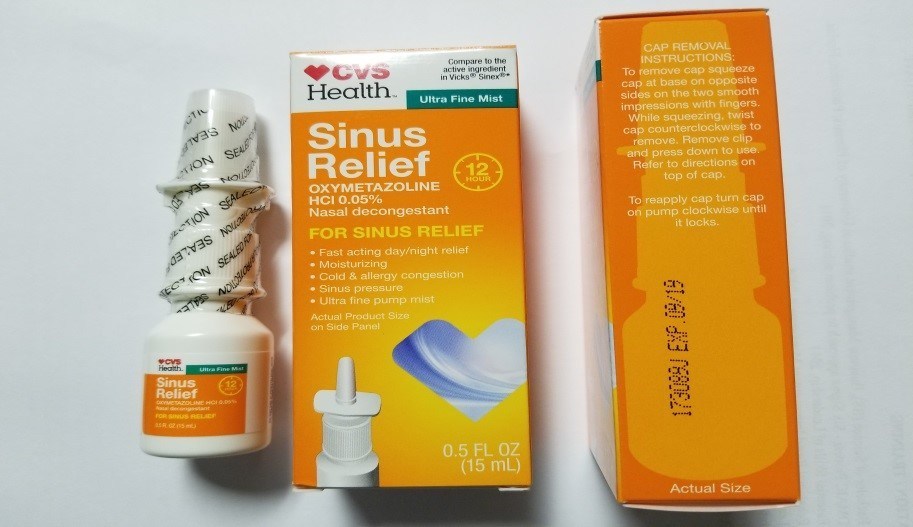
Generic drug maker Product Quest Manufacturing is recalling CVS Health 12 Hour Sinus Relief Nasal Mist after the product was found to be contaminated with Pseudomonas aeruginosa. While the recall affects just one batch of the nasal spray (Lot# 173089J), use of the contaminated product could result in a potentially life-threatening infection.
A representative from Product Quest declined to comment on the recall.
According to Product Quest, the company has not yet received any adverse event reports from customers who used the contaminated batch of nasal spray. Still, the affected batch of the over-the-counter (OTC) nasal spray has the potential to cause serious harm to allergy sufferers.
“Repetitive use of a nasal spray containing a gram-negative pathogen can potentially lead to colonization and subsequent infection which can be life threatening in certain patient populations, such as those with cystic fibrosis or immuno-compromised,” said the company in a statement about the recall.
This recall affects nearly 17,000 units of CVS Health 12 Hour Sinus Relief Nasal Mist which were sold in 0.5 fluid ounce bottles with an expiry date of September 2019. Product Quest reported that the nasal spray was distributed and sold at CVS drugstores across the US.
Customers, distributers and retailers are being notified of the recall by both oral and written communication and Product Quest is urging any consumers in possession of the affected batch to discontinue use of the nasal spray immediately. The company will also be offering to return or replace the product for customers.
However, the recall issued by the Florida-based drug manufacturer leaves many unanswered questions. For one, Product Quest didn’t disclose the source of the Gram-negative pathogen and how the contaminated batch was identified. No word yet on whether other batches of the nasal spray will be affected by the recall, but the company is conducting the recall with the knowledge of the US Food and Drug Administration (FDA).
This recall isn’t the first manufacturing problem the company has faced in recent months. In April, Product Quest received a warning letter from the FDA after a 2017 inspection of their North Carolina drug manufacturing facility identified violations of current good manufacturing practice (CGMP) regulations.
The infractions included poor cleaning procedures for manufacturing equipment and failure to investigate potential contamination of drug products. One of the most serious violations involved the side-by-side manufacturing of drug products destined for human use and hazardous pesticides.
“Your firm manufactured topical human drugs and several pesticides in the same building, using shared equipment,” said the FDA in its warning letter. “It is unacceptable as a matter of CGMP to continue manufacturing drugs using the same equipment that you use to manufacture pesticides or other non-pharmaceutical products due to the risk of cross-contamination.”
It’s clear that Product Quest’s manufacturing practices will need to updated in order to avoid such instances of drug contamination in the future.









Join or login to leave a comment
JOIN LOGIN