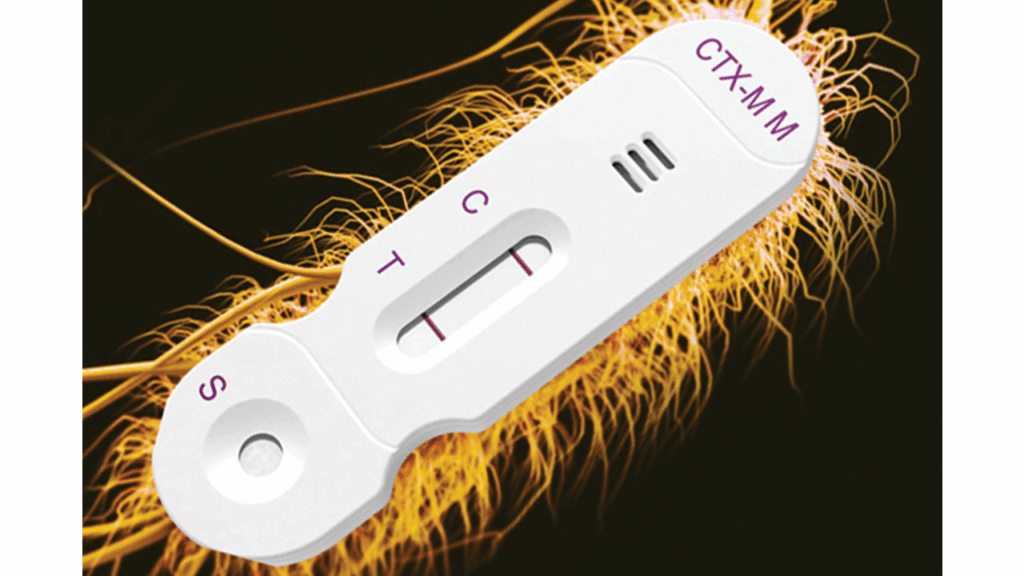
The FDA has cleared Hardy Diagnostics’ NG-TEST CTX-M Multi, a visual, in vitro, immunoassay that detects resistance-related enzymes in bacteria. The test identifies CTX-M enzymes — specifically groups 1, 2, 8, 9 and 25 — from pure colonies of Enterobacterales that may be producing extended-spectrum β-lactamases (ESBLs).
Enterobacterales, which include Escherichia coli (E.coli) and Klebsiella pneumoniae, are common causes of urinary tract and bloodstream infections. Some strains produce ESBLs, enzymes that break down widely used antibiotics and complicate treatment.
CTX-M enzymes were first reported in Germany in 1989 and have since become the most common ESBLs worldwide, including in the US. These enzymes are now frequently found in species like E. coli, Klebsiella pneumoniae and Proteus mirabilis, many of which are linked to hospital-acquired and drug-resistant infections.
XTALKS WEBINAR: IVD Studies: Steps for Success in Global Clinical Performance
Live and On-Demand: Thursday, November 13, 2025, at 11am EST (5pm CET/EU-Central)
Register for this free webinar to learn how global in vitro diagnostic (IVD) studies can meet regulatory expectations while maintaining safety and data integrity.
New Infections, Old Antibiotics and a Growing Resistance Challenge
CTX-M enzymes degrade beta-lactam antibiotics like penicillins and cephalosporins, making the bacteria that produce them more difficult to treat. In 2003, the CDC detected CTX-M enzymes in E. coli isolates across several states, signaling their growing footprint in North America.
ESBL-related infections often require longer treatment, drive up healthcare costs and limit antibiotic options. Rapid detection is increasingly vital as ESBL rates climb in both hospital and community settings.
A 2024 study of outpatient data from the US Veterans’ Health Administration found that community-acquired ESBL-producing E. coli rose from 4.1% in 2010 to 9.0% in 2019, with some counties exceeding 20%.
In 2017 alone, the CDC estimated over 197,000 ESBL-related infections in hospitalized patients across the US, resulting in more than 9,000 deaths.
Among ESBLs, CTX-M enzymes have become the most widespread subtype globally and in the US.
Variants such as CTX-M-15 and CTX-M-14 have become dominant since the 2000s, driven by bacterial gene transfer, global travel and antibiotic use in agriculture. As these strains spread, the increased reliance on carbapenems, often considered last-resort antibiotics, has compounded the challenge of antimicrobial resistance.
What Makes the NG-TEST CTX-M Multi Stand Out?
Traditional detection methods such as minimum inhibitory concentration (MIC) testing, disk diffusion and PCR are accurate but often time-consuming or require specialized equipment.
The NG-TEST CTX-M Multi provides a visual result in about 15 minutes. Designed for use with lab-grown bacterial colonies, the test does not require molecular tools, making it easier to implement in routine microbiology workflows.
In addition to earlier findings, CDC surveillance from 2021 recorded more than 3,000 ESBL-producing Enterobacterales cases across six US sites. Nearly 30% of these required hospitalization, and over 10% of hospitalized patients either died during their stay or within 30 days of diagnosis.
Nearly half of the cases had prior healthcare exposure, most often recent hospital admissions or surgeries. One-third of all cases involved isolates from urine specimens, a common infection site for ESBL-producing bacteria.
Hardy Diagnostics is the exclusive distributor of the NG-TEST CTX-M Multi in the US and its territories. The new test expands its diagnostic portfolio to support faster, targeted treatment decisions in response to the growing burden of antimicrobial resistance.
The global lateral flow assays market surpassed $9.8 billion in 2024. This growth is especially notable in North America and the US market, which is over $4.6 billion. Kits and reagents continue to be the leading product segment, reiterating the increasing investment in quick and accessible diagnostics.
If you want your company to be featured on Xtalks.com, please email [email protected].










Join or login to leave a comment
JOIN LOGIN