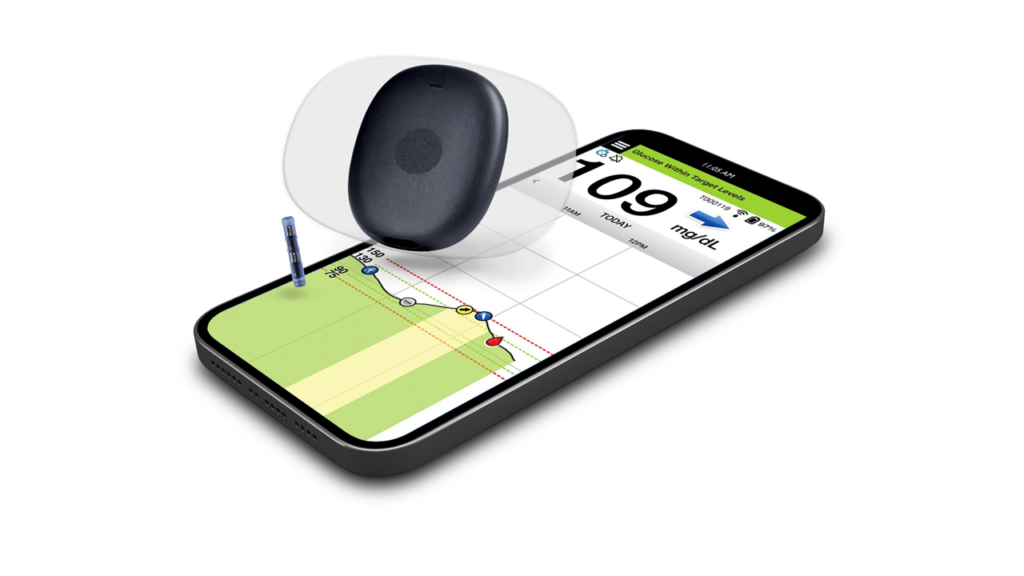
Senseonics Holdings, Inc., in partnership with Ascensia Diabetes Care, has secured US Food and Drug Administration (FDA) clearance for the Eversense 365 Continuous Glucose Monitoring (CGM) system, marking a pivotal moment in diabetes care.
As the world’s first 365-day CGM system, it redefines convenience for patients with type 1 and type 2 diabetes, offering CGM with just one sensor insertion each year. Unlike short-term CGM systems that require regular sensor swaps every 10 to 14 days, Eversense 365 eliminates these frequent interruptions, providing a seamless experience.
The sensor, implanted beneath the skin, remains secure, potentially avoiding the detachment issues that can occur with adhesive-based short-term CGM systems. Over the course of a year, patients can trust the system’s accuracy, with fewer false alarms — especially at night. The transmitter is removable, allowing users flexibility without sacrificing the sensor’s performance or needing a new warm-up phase, a feature unique to Eversense 365.
Cleared as an integrated continuous glucose monitoring (iCGM) system, Eversense 365 integrates effortlessly with automated insulin delivery (AID) systems. This makes it a powerful choice for those seeking more control and fewer disruptions in managing their diabetes. With discreet vibration alerts and real-time data sent every five minutes to a mobile app, the system ensures continuous, reliable monitoring.
XTALKS WEBINAR: Medical Device Safety: The Next Frontier
Live and On-Demand: Wednesday, October 23, 2024, at 1pm EDT (10am PDT)
Register for this free webinar to discover how to improve medical device safety by streamlining the development and regulatory process for medical devices.
Compared to other CGM systems launched in 2024, such as the Dexcom Stelo, a first-of-its-kind over-the-counter (OTC) glucose biosensor, and Abbott’s FreeStyle Libre, which recently integrated with Medtronic’s insulin delivery systems, Eversense 365 stands out for its year-long sensor life. While Dexcom’s Stelo prioritizes accessibility through OTC availability and FreeStyle Libre focuses on connectivity with insulin pumps, neither currently offer the long-term convenience of Eversense 365’s year-long sensor.
Earlier in the second quarter of 2024, Senseonics reported a revenue increase of more than 18 percent year-over-year, driven by the success of their existing Eversense systems. Senseonics’ potential collaboration with Mercy, a US healthcare system, aimed at integrating Eversense systems with remote patient monitoring (RPM) services, could improve clinical outcomes and reduce healthcare costs.
Senseonics also established Eon Care Services to streamline Eversense insertions and provide hands-on training for healthcare providers, a move that could likely boost access and adoption of the 365-day sensor in clinics.
Looking ahead, Senseonics is pushing forward with the Gemini System, a fully implantable, self-powering glucose sensor for type 2 diabetes, further expanding their CGM portfolio.










Join or login to leave a comment
JOIN LOGIN