The availability of anonymized, real-world clinical data sources (e.g. Medical Claims, Lab Results, and EMRs) is driving a paradigm shift in the understanding of the efficacy of medical treatments. Life Sciences companies are performing advanced analytics, including cohort and predictive analytics, on top of these Real-World Evidence (RWE) data sources to understand the performance of their drugs and devices when used by actual, non-clinical trials patients. Some of the most common RWE Analytics Use Cases include:
- Comparing treatment effectiveness
- Negotiating drug prices based on outcomes
- Increasing patient medication adherence
However, for many conditions requiring specialty clinical care, the era of obtaining RWE insights from insurance claims is drawing to a close. Life Sciences companies are developing treatments for increasingly smaller subsets of disease populations, expanding the information needed to define populations and characterize their course of illness. The data elements required to establish these patient populations and to demonstrate that new treatments improve patient outcomes relative to the usual care will require a new breed of technologies and tools. Fortunately, recent advancements made in Silicon Valley are shaping the path for the future of Real-World Evidence, where evidence generation is specialty-focused, efficient, and democratized
- With the emergence of the Internet of Things (IOT), future RWE data sources will become more diverse and comprehensive in measuring various aspects of patient health
- Blockchain technology, which debuted with Bitcoin and is now being further developed for healthcare, will enable the secure storage of patient health data and the direct transmission of anonymized data to Pharmaceutical companies
- Continued progress in Cognitive Computing will advance the analytic pipeline, enabling deeper insights to be gained from the stores of RWE data
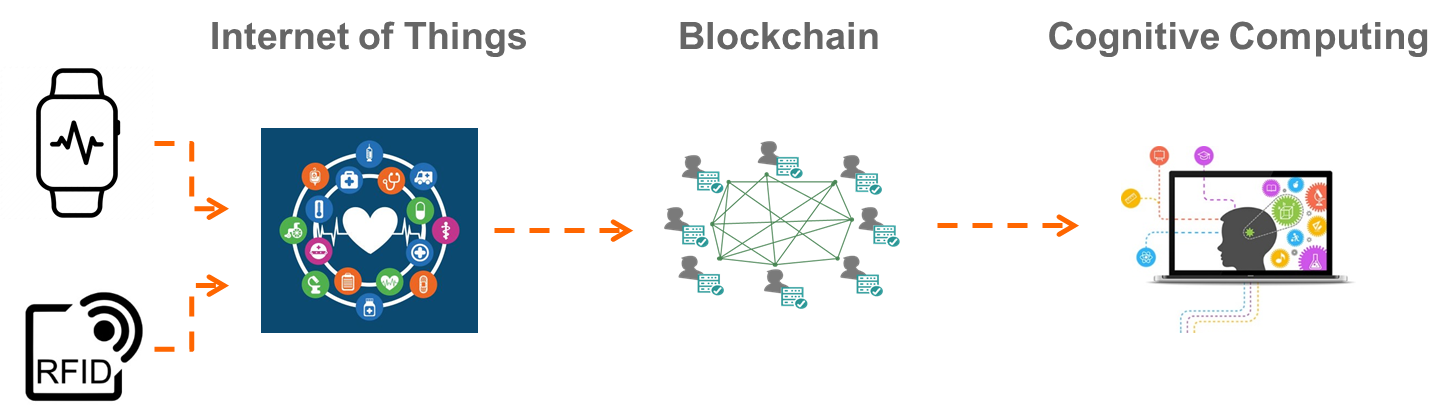
Modern advancements in data and analytics technologies represent an important milestone in evidence generation. Knowledgent’s experience in RWE research substantiates the notion that adoption of these newer technologies and management of exponentially growing real-world data sources will inevitably require the Life Sciences industry to standardize to a Common Data Model (CDM) to enable interoperable, transparent evidence generation ecosystem. In the future, data providers, technology providers, researchers, and other RWE stakeholders will increase the value of analytics in Life Sciences innovation by plugging into a standardized RWE infrastructure that supports interoperability between rich and diverse data sources and analytic tools.
To learn more about real-world evidence, register for the upcoming webinar hosted by Knowledgent and SAS.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.










Join or login to leave a comment
JOIN LOGIN