As oncology continues to dominate the pharmaceutical market, the global demand for effective cancer therapies has reached unprecedented levels. With advances in precision medicine and immunotherapy driving innovation, leading oncology drugs are not only transforming treatment paradigms but also generating billions in revenue. The year 2023 was no exception, with several cancer drugs achieving remarkable sales milestones, reflecting their efficacy, expanding indications and the growing global cancer burden.
In this blog, we highlight the top 10 cancer drugs to watch in 2024, based on their impressive 2023 sales figures and anticipated market dynamics. From groundbreaking checkpoint inhibitors like Keytruda and Opdivo to targeted therapies such as Tagrisso and Ibrance, these drugs represent the cutting edge of oncology. With patent exclusivities, new approvals and evolving treatment protocols shaping their trajectories, these therapies are poised to remain critical players in the fight against cancer.
Read on to learn what makes these drugs indispensable in oncology, the factors driving their commercial success and their potential to influence the future of cancer treatment.
Note: When it comes to companies that report in foreign currencies, the conversion to US dollars uses the average annual exchange rates reported by the US Federal Reserve.
1. Keytruda (Pembrolizumab)
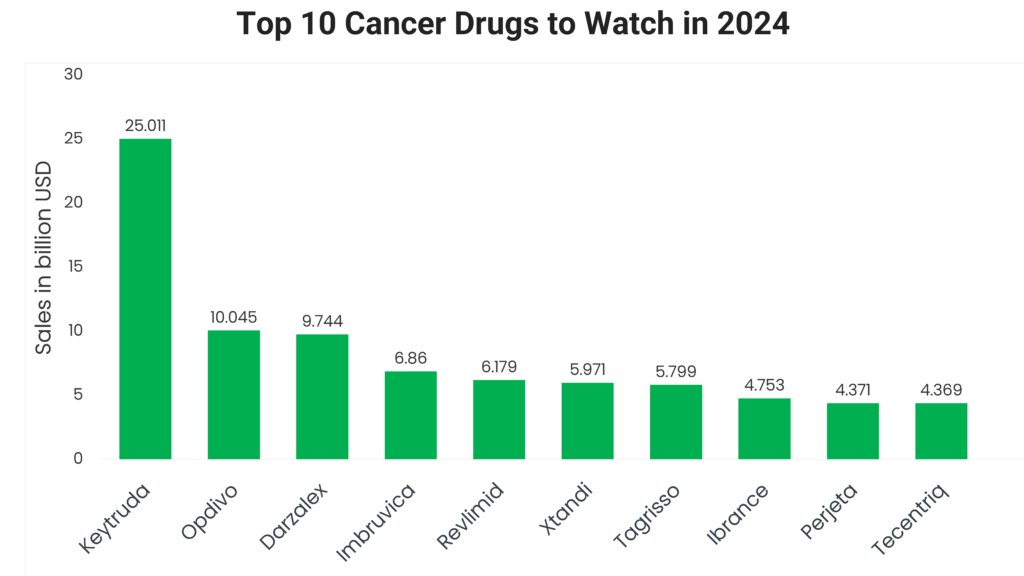
Keytruda 2023 sales: $25.011 billion
Company/developer: Merck
Date of first US Food and Drug Administration (FDA) approval: September 4, 2014
Indications Keytruda is FDA-approved for: Unresectable or metastatic melanoma. Keytruda is specifically indicated for the adjuvant treatment of adult and pediatric (12 years and older) patients with stage IIB, IIC or III melanoma following complete resection. It is also approved to treat non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), classical Hodgkin lymphoma, primary mediastinal large B-cell lymphoma (PMBCL), urothelial carcinoma, microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) cancer, MSI-H or dMMR colorectal cancer, gastric cancer, esophageal cancer, cervical cancer, hepatocellular carcinoma, biliary tract cancer, Merkel cell carcinoma, renal cell carcinoma, endometrial carcinoma, tumor mutational burden-high (TMB-H) cancer, cutaneous squamous cell carcinoma (cSCC) and triple-negative breast cancer.
List price of Keytruda: As stated on Keytruda’s website, the list price for a single dose of the medication is $11,564.16 when administered every three weeks. For dosing every six weeks, the cost is $23,138.32.
Why it sold so well: In 2023, Keytruda claimed the top spot as the world’s best-selling drug, a milestone achieved after AbbVie’s Humira (adalimumab) faced patent expiration and competition from biosimilars. Already a leading oncology drug, Keytruda saw a 21 percent increase in sales in 2023, reaching $25 billion at constant exchange rates.
The drug also secured nearly half a dozen new FDA approvals for oncology indications. Among the most significant was its approval as a neoadjuvant-adjuvant therapy around surgery for resectable NSCLC, making it the first PD-1/L1 inhibitor regimen cleared for both pre- and post-surgery use. Additionally, it was approved as a first-line treatment in combination with Astellas and Pfizer’s Padcev (enfortumab vedotin) for locally advanced or metastatic urothelial cancer, and for use alongside chemotherapy as a first-line treatment for locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma.
However, Keytruda’s position as the top-selling drug may be short-lived. Glucagon-like peptide-1 (GLP-1) agonists, popular for treating type 2 diabetes and obesity, are rapidly gaining traction. In 2023, Novo Nordisk’s semaglutide products, including Ozempic, Wegovy and Rybelsus, generated sales of 145.8 billion DKK (approximately $21.1 billion USD). Meanwhile, Eli Lilly’s Mounjaro (tirzepatide), a dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 agonist, brought in $5.2 billion, and its obesity-specific version, Zepbound, earned over $175 million in its first quarter on the market.
Keytruda is expected to maintain its strong market presence for a few more years, with its patent exclusivity set to expire in 2028.
2. Opdivo (Nivolumab)
Opdivo 2023 sales: $10.045 billion
Company/developer: Bristol Myers Squibb (BMS) and Ono Pharmaceutical
Date of first FDA approval: December 22, 2014
Indications Opdivo is FDA-approved for: Melanoma, metastatic NSCLC, renal cell carcinoma, Hodgkin’s lymphoma, head and neck cancer, urothelial carcinoma, colorectal cancer, hepatocellular carcinoma, esophageal carcinoma, malignant pleural mesothelioma, gastric cancer
List price of Opdivo: The list price for a combined infusion of Opdivo at 3 mg/kg and Yervoy at 1 mg/kg is $25,041, while the price for an infusion of Opdivo at 3 mg/kg alone is $7,635.
Why it sold so well: In 2023, Opdivo generated $10 billion in global sales, with $9.009 billion attributed to BMS and 145.5 billion Japanese yen (approximately $1.036 billion USD) earned by Ono Pharmaceutical.
As BMS’s second-highest revenue-generating product, the PD-1 inhibitor continued its growth trajectory in 2023. However, it remains outpaced by its competitor Keytruda, which became the top-selling drug worldwide that year. While Keytruda has achieved numerous label expansions, Opdivo’s growth has been more incremental. In March 2024, Opdivo received FDA approval for use alongside chemotherapy as a first-line treatment for bladder cancer, similar to Keytruda’s approval in combination with Padcev for the same indication.
Although Opdivo’s patent exclusivity will expire in 2028, BMS is actively pursuing additional opportunities. Phase III trials are underway to evaluate Opdivo in muscle-invasive urothelial carcinoma, hepatocellular carcinoma (HCC) and periadjuvant NSCLC. The company is also developing a subcutaneous formulation of the drug. While Merck is working on a similar version for Keytruda, BMS is further along and may secure approval first. If successful, Opdivo could become the first PD-1/L1 inhibitor available in a subcutaneous formulation in the US. BMS aims to convert 30 percent to 40 percent of the US Opdivo market to this new injectable form.
3. Darzalex (Daratumumab)
Darzalex 2023 sales: $9.744 billion
Company/developer: Janssen Biotech, Inc. (part of Johnson & Johnson) in collaboration with Genmab
Date of first FDA approval: November 16, 2015, for Darzalex; May 1, 2020, for Darzalex Faspro
Indications Darzalex/Darzalex Faspro are FDA-approved for:
- Darzalex: As an intravenous injection into the vein for multiple myeloma treatment in various stages (newly diagnosed and those who have received one or more prior lines of therapy), for patients both eligible and ineligible for autologous stem cell transplant and as a monotherapy in pre-treated patients.
- Darzalex Faspro: As a subcutaneous injection in the abdomen for multiple myeloma across similar settings as Darzalex, including for both autologous stem cell transplant-eligible and transplant-ineligible patients.
Price of Darzalex and Darzalex Faspro:
- Darzalex: Intravenous solution at a concentration of 20 mg/mL, priced starting at $783.81 for a 5 mL vial.
- Darzalex Faspro: Subcutaneous solution containing 1,800 mg and 30,000 units per 15 mL, priced from $10,563.33 for a 15 mL vial.
Why they sold so well: Multiple myeloma, the second most common type of blood cancer, remains incurable, with current treatments aimed at extending survival and improving patients’ quality of life. In 2024, over 35,000 new cases are expected to be diagnosed in the US.
Darzalex and Darzalex Faspro have transformed multiple myeloma treatment by targeting the CD38 molecule, which is abundantly expressed on the surface of myeloma cells. This innovative mechanism not only directly kills cancer cells but also enhances the immune system’s anti-tumor response. Darzalex Faspro, with its subcutaneous administration, offers a faster and potentially less painful alternative to intravenous infusions, improving patient comfort and adherence.
These advantages, along with strong clinical trial data, have driven rapid adoption in practice. Notable studies such as PERSEUS, MAIA and CASSIOPEIA have demonstrated significant benefits, including improved progression-free survival (PFS) and high overall survival rates in patients receiving Darzalex-based regimens.
In the first quarter of 2024, global net sales for Darzalex and Darzalex Faspro reached $2.692 billion, with $1.464 billion generated in the US and $1.228 billion from other markets. Genmab earns royalties from Janssen Biotech, Inc. on these sales under an exclusive licensing agreement that allows Janssen to develop, produce and market daratumumab.
In 2023, Darzalex sales rose to $9.744 billion, a 22 percent increase compared to the previous year, driven by expanding market share worldwide. This growth trajectory is expected to continue, bolstered by the therapy’s proven efficacy, expanding indications and convenient treatment options.
Both Janssen and Genmab anticipate sustained growth for Darzalex and Darzalex Faspro, given their established roles in multiple myeloma care and potential for broader use in evolving treatment protocols.
4. Imbruvica (Ibrutinib)
Imbruvica 2023 sales: $6.860 billion
Company/developer: LLC (an AbbVie Company) and Janssen Biotech Inc.
Date of first FDA approval: November 13, 2013
Indications Imbruvica is FDA-approved for: Imbruvica is approved for several conditions, including chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), Waldenström’s macroglobulinemia (WM) and chronic graft-versus-host disease (cGVHD) after failure of at least one prior systemic therapy. Initially, Imbruvica received approval for mantle cell lymphoma (MCL) and marginal zone lymphoma (MZL) based on Phase II studies. However, these approvals were recently withdrawn after the Phase III SHINE and SELENE trials failed to meet their primary endpoints. Despite this, Imbruvica remains approved for relapsed/refractory (R/R) MCL in more than 100 countries and for R/R MZL in over 30 countries, supported by positive outcomes from earlier clinical trials.
Price of Imbruvica: Imbruvica is offered in several formulations, with wholesale acquisition costs (WAC) starting at $10,210.73 for a 70 mg/mL oral suspension and going up to $22,690.52 for a 120-count package of 140 mg capsules.
Why it sold so well: Imbruvica is a kinase inhibitor that targets Bruton’s tyrosine kinase (BTK), a crucial protein in the B-cell receptor signaling pathway. This protein plays a vital role in the survival, growth and movement of malignant B cells. By inhibiting BTK, Imbruvica disrupts these processes, helping to slow or prevent the spread of cancer cells.
In 2023, Statista ranked Imbruvica among the top ten oncology products worldwide. Despite operating in a competitive market, the drug remained a major contributor to AbbVie’s oncology portfolio, which recorded $5.915 billion in full-year net revenues. Imbruvica alone generated $3.596 billion for AbbVie, a significant figure though 21.3 percent lower than the previous year due to increased competition and changing market conditions. Janssen Biotech also reported $3.264 billion in revenue from Imbruvica in 2023.
In the fourth quarter of 2023, Imbruvica earned $903 million for AbbVie, a 19 percent decline compared to the same period the previous year. The US market remains the largest revenue source, contributing $683 million, highlighting the drug’s continued importance within AbbVie’s product lineup despite the downward trend.
Looking ahead to 2024, AbbVie aims to leverage acquisitions and implement strategic initiatives to strengthen and stabilize Imbruvica’s position in the oncology market, mitigating the impact of competition and market pressures.
5. Revlimid (Lenalidomide)
Revlimid 2023 sales: $6.179 billion
Company/developer: BMS
Date of first FDA approval: December 27, 2005
Indications Revlimid is FDA-approved for: Revlimid, a thalidomide derivative, is approved for use in adult patients to treat multiple myeloma in combination with dexamethasone. It is also indicated for the treatment of myelodysplastic syndromes, MCL, follicular lymphoma and MZL.
Price of Revlimid: The cost for a 28-capsule supply of Revlimid (2.5 mg) is around $26,296.
Why it sold so well: Revlimid has long been a leading first-line treatment for multiple myeloma and a top performer for BMS, benefiting from its position as the sole treatment option for many years. Its patent protection allowed it to dominate the market for over a decade. However, in 2022, its patent expired, and the introduction of generic alternatives led to a decline in sales for BMS last year.
6. Xtandi (Enzalutamide)
Xtandi 2023 sales: $5.971 billion
Company/developer: Pfizer/Astellas Pharma
Date of first FDA approval: August 31, 2012
Indications Xtandi is FDA-approved for: Prostate cancer that remains localized and has not metastasized to other areas. Xtandi is also prescribed for metastatic prostate cancer that continues to respond to hormone therapy.
List price of Xtandi: $14,322.47 per month.
Why it sold so well: Xtandi, initially approved by the FDA in 2012 for metastatic castration-resistant prostate cancer (mCRPC), has become a key treatment option across multiple stages of prostate cancer. In 2018, it received approval for non-metastatic castration-resistant prostate cancer, followed by approval in 2019 for metastatic castration-sensitive prostate cancer.
In November 2023, Xtandi was approved for non-metastatic castration-sensitive prostate cancer with biochemical recurrence at high risk of metastasis. This latest approval makes Xtandi the first androgen receptor signaling inhibitor approved for use without gonadotropin-releasing hormone therapy in non-metastatic castration-sensitive prostate cancer.
Xtandi continues to generate substantial revenue and remains protected by US patents until August 2027, preventing competition from generic alternatives.
7. Tagrisso (Osimertinib)
Tagrisso 2023 sales: $5.799 billion
Company/developer: AstraZeneca
Date of first FDA approval: November 13, 2015
Indications Tagrisso is FDA-approved for: Tagrisso is a prescription medication used to treat adults with NSCLC that involves specific mutations in the epidermal growth factor receptor (EGFR) gene.
Price of Tagrisso: The price for a 30-tablet supply of Tagrisso 40 mg oral tablets is approximately $18,034.
Why It sold so well: Tagrisso is a third-generation, irreversible EGFR-tyrosine kinase inhibitor (TKI) recognized for its effectiveness in treating NSCLC, including cases with metastases to the central nervous system. It is available in 40 mg and 80 mg oral tablets, taken once daily, and has been used by over 800,000 patients worldwide for its approved indications.
AstraZeneca continues to explore Tagrisso’s potential for treating patients at various stages of metastatic EGFR-mutated NSCLC. The drug is approved as a standalone therapy in more than 100 countries, including the US, the European Union (EU), China and Japan.
8. Ibrance (Palbociclib)
Ibrance 2023 sales: $4.753 billion
Company/developer: Pfizer Inc.
Date of first FDA approval: February 3, 2015
Disease(s) it is FDA-approved to treat: Ibrance is a kinase inhibitor used for treating hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer. It is prescribed in combination with an aromatase inhibitor as initial endocrine therapy for postmenopausal women or with fulvestrant for women whose disease has progressed after previous endocrine therapy.
Price of Ibrance: The cost of Ibrance 75 mg oral capsules is approximately $16,839 for a supply of 21 capsules.
Why it sold so well: Ibrance achieved over $1.11 billion in global sales in 2023. In Phase III clinical trials, Ibrance demonstrated significant benefits as a first-line treatment for adults with HR-positive, HER2-negative metastatic breast cancer, showing strong progression-free survival data. The combination of Ibrance and letrozole delayed disease progression for a median of 24.8 months, compared to 14.5 months for those receiving letrozole and a placebo. Patients treated with Ibrance and letrozole experienced a 42 percent reduction in the risk of disease progression compared to those on letrozole and placebo.
9. Perjeta (Pertuzumab)
Perjeta 2023 sales: $4.371 billion
Company/developer: Genentech (Roche)
Date of first FDA approval: June 8, 2012
Disease(s) it is FDA-approved to treat: Perjeta is a humanized monoclonal antibody specifically designed for the treatment of HER2-positive breast cancer. It is administered in combination with Herceptin, another targeted therapy medicine, and Taxotere, a type of chemotherapy. This combination is used to address HER2-positive metastatic breast cancer in patients who have not undergone prior treatment with either Herceptin or chemotherapy.
Price of Perjeta: Perjeta carries a monthly cost of $5,900, totaling approximately $71,000 annually.
Why it sold so well: The FDA’s approval is based on the Phase III APHINITY study, which showed that adjuvant treatment with Perjeta, Herceptin and chemotherapy significantly reduced the risk of invasive breast cancer recurrence or mortality compared to Herceptin and chemotherapy alone. Perjeta’s sales reached $4.3 billion in 2023, reflecting a 1 percent increase, with international markets, particularly China and Brazil, driving much of the growth.
10. Tecentriq (Atezolizumab)
Tecentriq 2023 sales: $4.369 billion
Company/developer: Genentech, Inc.
Date of first FDA approval: May 18, 2016
Disease(s) it is FDA-approved to treat: Tecentriq is a monoclonal antibody designed to bind to the PD-L1 protein expressed on tumor cells and tumor-infiltrating immune cells, blocking its interaction with both PD-1 and B7.1 receptors. By inhibiting PD-L1, Tecentriq may facilitate the activation of T cells. It is approved in the US, EU, and various other countries for use alone or in combination with targeted therapies and/or chemotherapies to treat various forms of metastatic NSCLC, small cell lung cancer (SCLC), hepatocellular carcinoma (HCC), certain types of metastatic urothelial cancer, PD-L1-positive metastatic triple-negative breast cancer, and advanced melanoma with a BRAF V600 mutation.
Price of Tecentriq: The cost for atezolizumab is $9,035 per 28-day.
Why it sold so well: Tecentriq treatment led to significantly longer overall survival compared to platinum-based chemotherapy in patients with NSCLC who had high PD-L1 expression. In a clinical trial involving 205 individuals with metastatic NSCLC, who tested positive for high PD-L1 and had no abnormal EGFR or ALK genes, those treated with Tecentriq lived longer (20.2 months) compared to those receiving platinum-based chemotherapy (13.1 months). Tecentriq achieved over $4,275 billion in global sales in 2023, marking a 9% increase from 2022.











Join or login to leave a comment
JOIN LOGIN