As technology continues to revolutionize every sector of our lives, the medical device industry stands at the forefront of this innovation, playing a pivotal role in enhancing patient care, improving diagnostic accuracy and transforming treatment modalities. This article takes a comprehensive look at the top 10 medical device companies that are leading the charge in this rapidly evolving field.
These powerhouse organizations have not only distinguished themselves through their cutting-edge devices and technologies but also via their robust research and development (R&D) capabilities, unwavering commitment to quality and extensive global reach. From advanced diagnostic equipment to innovative therapeutic devices, these industry titans are shaping the future of healthcare and paving the way for unprecedented medical breakthroughs.
As we delve into the accomplishments, unique offerings and strategic developments of each company, we will also highlight their respective strategies to tackle the dynamic and increasingly complex global healthcare landscape. Join us as we explore the inspiring journey and significant contributions of these top 10 medical device companies.
Note: When it comes to companies that report in foreign currencies, the conversion to U.S. dollars utilizes the average annual exchange rates reported by the US Internal Revenue Service (IRS).
RELATED: Top 30 Pharma and Biotech Companies in 2023: Statistics and Trends

1) Medtronic
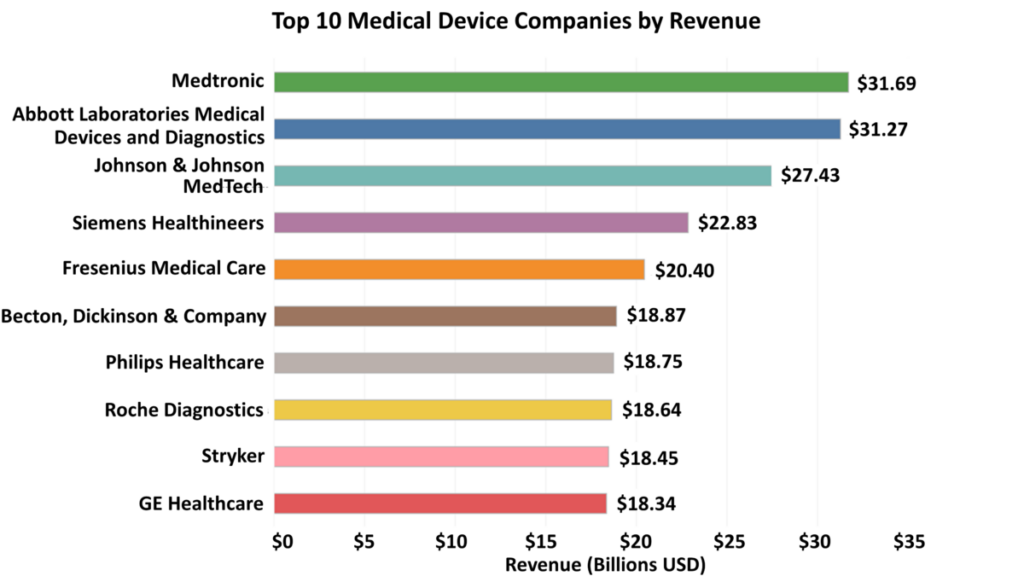
2022 Revenue: Medtronic reported an annual revenue of $31.69 billion for the fiscal year ending April 29, 2022, a 5.21 percent increase from its 2021 fiscal year revenue of $30.12 billion.
2022 Net Income: Medtronic’s net income was $5.04 billion for the fiscal year ending April 29, 2022, a 39.74 percent increase from its net income of $3.61 billion in the 2021 fiscal year.
2022 R&D Spend: Medtronic’s R&D expenses were $2.75 billion for the fiscal year ending April 29, 2022, a 10.15 percent increase from the $2.49 billion spent on R&D in the 2021 fiscal year.
Medtronic, the leading global medical device manufacturer, reported a revenue growth of 5 percent in the last fiscal year, affirming its position as the top global medical technology company by revenue. The growth is attributed to regulatory approvals, strategic partnerships and the acquisitions of innovative medical technology companies. Medtronic’s proactive stance towards R&D is evident from the over 230 clinical trials it conducted and the more than 200 regulatory approvals it received in the US, Europe, Japan and China in the 2022 fiscal year. The company reported an investment of $2.7 billion in R&D in the 2022 fiscal year, which significantly contributed to its growth.
In August 2022, Medtronic acquired Affera, a company that develops and manufactures cardiac mapping and navigation systems as well as catheter-based cardiac ablation technologies. This acquisition bolstered Medtronic’s position in the domain of cardiac ablation solutions. In Medtronic’s annual report, CEO Geoff Martha attributes their success in this area to their comprehensive suite of products and solutions, which equip clinicians with the tools necessary for optimal atrial fibrillation care.
The US Food and Drug Administration (FDA) granted approval to four of Medtronic’s cardiovascular product offerings, further expanding the company’s foothold in this sector. This includes an expanded approval for the Arctic Front family of Cardiac Cryoablation Catheters, clearance for two AccuRhythm AI algorithms designed for use with cardiac monitors, approval for a self-expanding transcatheter aortic valve replacement system and expanded approval for the Freezor and Freezor Xtra Cardiac Cryoablation Focal Catheters.
The FDA also gave clearance to several products in the neuroscience segment, including the SenSight Directional Lead system used for deep brain stimulation (DBS) therapy and Vanta, a high-performance, recharge-free implantable neurostimulator with a lifespan of up to 11 years. Other approvals included the Intellis rechargeable neurostimulator for treating chronic pain associated with diabetic peripheral neuropathy (DPN).
Medtronic’s Hugo Robotic-Assisted Surgery system, released in a limited market in June 2021, has been receiving positive reviews for its performance in various surgeries. In addition, Medtronic partnered with Vizient, a healthcare performance improvement company, to include Medtronic’s touch surgery enterprise in Vizient’s product catalog across the US.
Medtronic has also embarked on a partnership with GE Healthcare, concentrating on the specific requirements and care demands at Ambulatory Surgery Centers (ASCs) and Office-Based Labs (OBLs).
Internationally, Medtronic expanded its MiniMed 780G insulin pump and Guardian 4 sensor. It also plans to launch these products in the US.
The company is also focusing on developing its Symplicity Spyral Renal Denervation (RDN) system for achieving target blood pressure level ranges and is conducting a couple of clinical studies with the system. Three-year data from one of their clinical studies showed that RDN potentially doubles the duration patients remain within their desired blood pressure range, irrespective of their use of anti-hypertensive medications. Medtronic is optimistic about this opportunity and anticipates high demand across a large global market.
Subsequent to fiscal year 2022, on May 13, 2022, Medtronic acquired Intersect ENT, a global leader in ear, nose and throat (ENT) medical technology. This acquisition broadens Medtronic’s portfolio of ENT procedure products, creating a more comprehensive suite of solutions for surgeons treating patients with chronic rhinosinusitis (CRS).
On May 25, 2022, Medtronic and DaVita Inc. agreed to form a new, independent kidney care-focused medical device company (NewCo), in which they will have equal equity ownership. The transaction, which involves Medtronic contributing its entire Renal Care Solutions (RCS) business to NewCo, is anticipated to close in 2023, pending customary regulatory approvals and closing conditions. RCS currently falls under the Respiratory, Gastrointestinal and Renal division of Medtronic’s Medical Surgical portfolio.

2) Abbott Laboratories (Medical Devices and Diagnostics Divisions)
2022 Revenue: Abbott Laboratories’ Medical Devices and Diagnostics divisions brought in $31.27 billion in annual revenue in 2022, a 4.20 percent increase from $30.01 billion in 2021.
2022 Net Income: Abbott Laboratories’ annual net income for 2022 was $6.93 billion, a 1.95 percent decline from $7.07 billion in 2021.
2022 R&D Spend: In 2022, Abbott Laboratories spent $2.89 billion on R&D, a 5.32 percent increase from $2.74 billion in 2021.
Abbott Laboratories reported that the sales from its Diagnostics and Medical Device divisions in 2022 surpassed the company’s initial forecast. Abbott’s primary focus is on diagnostics and medical devices, but it also has nutrition and pharmaceutical divisions. The remarkable performance in 2022 was driven largely by the sales of COVID-19 testing kits, including the BinaxNOW COVID-19 Ag test and its at-home self-test version. Additionally, Abbott launched over 125 new products in 2022, in conjunction with a series of FDA approvals.
Key product introductions for Abbott last year included the FreeStyle Libre 3, which the company highlights as the world’s smallest and most accurate continuous glucose monitoring sensor. The impact of this innovative technology is expected to be further enhanced through a strategic partnership established with WeightWatchers in 2022, which integrates the FreeStyle Libre portfolio with a diabetes-specific weight management program, providing crucial health information and insights for individuals living with diabetes.
Another significant development was the introduction of the EnSite X EP system with EnSite Omnipolar Technology (OT), an innovative cardiac mapping platform by Abbott. This platform is designed to generate detailed 3D maps of the heart, assisting physicians in identifying and addressing the areas where abnormal rhythms originate.
Moreover, Abbott launched Aveir, a single-chamber leadless pacemaker for the treatment of patients with slow heart rhythms. This unique device is implanted directly inside the right ventricle of the heart through a minimally invasive procedure. It boasts a unique mapping capability for assessing correct positioning before placement and is designed to be fully retrievable.
The CardioMEMs HF system was another crucial launch for Abbott. This system is the first and only FDA-approved heart failure monitoring system that enables physicians to track changes in pulmonary artery pressure. Additionally, Abbott shared new clinical data indicating that its HeartMate 3 heart pump could significantly improve survival rates for patients with advanced heart failure, potentially extending life beyond five years. This is a significant breakthrough for patients who typically wouldn’t be expected to survive past a year. In the same vein, the company also broadened the distribution of its JETi thrombectomy device, designed for blood clot removal.
The company also launched Proclaim Plus, a spinal cord stimulation system featuring the next generation of Abbott’s proprietary BurstDR therapy, which received FDA approval. This advanced device offers pain coverage across up to six areas of the trunk and/or limbs, and provides physicians with the flexibility to adjust programming as a patient’s therapeutic needs evolve.
On the diagnostic front, Abbott secured FDA clearance for its pioneering Alinity m STI assay, which is capable of simultaneously detecting and differentiating up to four common sexually transmitted infections (STIs). Additionally, the FDA granted Emergency Use Authorization (EUA) for Abbott’s molecular test Alinity m MPXV for detecting the monkeypox virus (MPXV).
In Abbott Laboratories’ annual report, CEO Robert B. Ford attributes the company’s success to its diversified business strategy, which provides both defensive strength against market downturns and offensive strength by offering more competitive options. This strategy, he asserts, enables them to succeed even in the current challenging environment.

3) Johnson & Johnson MedTech
2022 Revenue: The sales of Johnson & Johnson’s MedTech segment were $27.43 billion in 2022, an increase of 1.36 percent from $27.06 billion in 2021.
2022 Net Income: The net income of Johnson & Johnson MedTech was $4.61 billion in 2022, a 5.35 percent increase from $4.37 billion in 2021.
2022 R&D Spend: In 2022, Johnson & Johnson MedTech spent $2.49 billion on R&D, a 4.67 percent increase from $2.38 billion in 2021.
The growth of Johnson & Johnson MedTech’s sales was propelled by the company’s commitment to innovation, reflected in its pipeline of more than 20 programs. The acquisition of Abiomed fortified its cardiovascular portfolio, which accounts for over $1 billion in annual revenue. Although the Surgery franchise experienced a 1.2 percent decline in sales in 2022 due to competitive pressures, the Orthopaedics franchise maintained its sales level from the prior year. Conversely, the Vision and Interventional Solutions franchises witnessed respective sales increases of 3.4 percent and 8.3 percent in 2022.
Acclarent Inc, a subsidiary of Johnson & Johnson MedTech, announced FDA clearance for the TruDi Shaver Blade. This single-use electromagnetically navigated blade, used with the Bien-Air S120 Shaver, facilitates the incision and removal of soft and hard tissue or bone in ENT, ENT skull base, maxillofacial surgery and head and neck surgery.
DePuy Synthes, the orthopedics company of Johnson & Johnson, secured FDA clearance for its Teligen system. This integrated technology platform enables minimally invasive surgical transforaminal lumbar interbody fusion (MIS-TLIF) procedures through digital tools for visualization and access. DePuy Synthes also got FDA clearance for the Altalyne Ultra Alignment system, designed for adolescent spinal deformities, such as scoliosis, and the Inhance Shoulder system for use in reverse total shoulder arthroplasty procedures.
Johnson & Johnson Vision, another division of Johnson & Johnson MedTech, received FDA approvals for Acuvue Oasys Max 1-Day and Acuvue Oasys Max 1-Day Multifocal contact lenses, as well as an expanded FDA approval for Acuvue Abiliti Overnight therapeutic lenses for myopia (nearsightedness). These approvals reinforced the company’s position as a global leader in eye health.
“Through our global infrastructure, robust understanding of diseases, cross-sector expertise, vast innovation network and trusted partnerships, J&J MedTech is maximizing our innovation impact to meet the challenges of today and forge new frontiers of health for tomorrow — from delivering more than 20 first-time major launches from our R&D pipeline; to bringing state-of-the-art digital and robotic solutions into the operating room; to elevating the skills and proficiency of HCPs [healthcare professionals] throughout the US; to creating greater agility, adaptability and resilience in our supply chain,” said Ashley McEvoy, executive VP and worldwide chairman of Johnson & Johnson MedTech, in the company’s news release.

4) Siemens Healthineers
2022 Revenue: Siemens Healthineers’ annual revenue from the year ended September 30, 2022, was €21.71 billion ($22.83 billion USD), a 20.65 percent increase from €18.00 billion ($21.27 billion USD) from the year ended September 30, 2021.
2022 Net Income: For the year ended September 30, 2022, Siemens Healthineers’ annual net income was €2.05 billion ($2.16 billion USD), a 17.64 percent increase from €1.75 billion ($2.06 billion USD) for the year ended September 30, 2021.
2022 R&D Spend: Siemens Healthineers’ R&D expenses from the year ended September 30, 2022, were €1.79 billion ($1.88 billion USD), a 15.46 percent increase from the €1.55 billion ($1.83 billion USD) spent on R&D during the year ended September 30, 2021.
Siemens Healthineers holds a commanding market position in numerous growth regions, boasting direct representation in over 70 countries worldwide. With primary production sites located in the US, China and Germany, the company designs, manufactures and markets a broad range of innovative diagnostic and therapeutic products and services to healthcare providers in upwards of 180 countries.
The firm made a robust start in 2022, driven by considerable diagnostics revenue alongside growth in imaging revenue. Early in 2022, they launched the Artis icono biplane, a novel system for the diagnosis and treatment of cardiac arrhythmia, coronary heart disease and structural heart disease. Numerous partnerships with hospitals and other medtech companies were also announced.
Siemens Healthineers further demonstrated its commitment to innovation at the European Congress of Radiology in Vienna, showcasing its revamped Acuson ultrasound portfolio.
The company strengthened its position by securing FDA approvals for its imaging and diagnostic technologies. The Imaging segment continued to be the leading revenue generator, followed by Diagnostics, Varian and Advanced Therapies, respectively.
During the 2022 fiscal year, Siemens Healthineers focused on projects such as integrating 3D imaging with endoluminal robotics for precise transbronchial lung biopsies. This integration, which incorporates Siemens Healthineers’ mobile 3D imaging system, Cios Spin, and Intuitive’s Ion Endoluminal system for transbronchial biopsies, enables automated 3D image transfers to update the target location of the lesion. Within the Imaging segment, the company developed and introduced the Naeotom Alpha CT system, a photon-counting computed tomography (CT) scanner based on an innovative sensor technology.
Siemens Healthineers also launched Mobilett Impact, its latest mobile X-ray system, in 2022. This system blends the benefits of a mobile X-ray system for bedside imaging with full digital integration, all offered at an economical price. Additionally, the company obtained FDA clearances for the Artis icono ceiling angiography system, Magnetom Free.Star 60cm MR scanner and Symbia Pro.specta SPECT/CT scanner.
As of September 30, 2022, Siemens Healthineers held approximately 23,000 technical intellectual property rights, including around 15,000 granted patents.

5) Fresenius Medical Care
2022 Revenue: Fresenius Medical Care’s annual revenue was €19.40 billion ($20.40 billion USD) in 2022, a 10.10 percent increase from €17.62 billion ($20.83 billion USD) in 2021.
2022 Net Income: In 2022, Fresenius Medical Care’s annual net income was €673 million ($708 million USD), a 30.55 percent decrease from €969 million ($1,145 million USD) in 2021.
2022 R&D Spend: In 2022, Fresenius Medical Care spent €229 million ($241 million USD) on R&D, a 3.62 percent increase from €221 million ($261 million USD) in 2021.
Fresenius Medical Care, a globally recognized provider of products and services for renal diseases, stands out for its publicly reported revenue and the volume of patients treated. The company’s top source of revenue is from dialysis care services, with the operation of 4,116 proprietary dialysis centers spanning roughly 50 countries. In addition to dialysis treatments, Fresenius Medical Care offers a range of healthcare services including value and risk-based care programs, pharmacy services and services related to vascular, cardiovascular and endovascular specialties, as well as ambulatory surgery centers.
Continually working towards the launch of new products, the company is innovatively expanding its portfolio. One such initiative is the digital therapy management platform, Kinexus, designed to support every automated peritoneal dialysis (APD) cycler in their roster. It is already available with the Liberty Select cycler, a peritoneal dialysis machine which received FDA clearance in November 2022. Fresenius Medical Care also plans to roll out Silencia, a novel APD cycler that enables high-quality ADP at a significantly reduced cost. In addition, the company received FDA approval at the start of this year for the 2008-series hemodialysis machines equipped with silicon tubing.
Throughout 2022, Fresenius Medical Care prioritized the digitalization of their services and enhancement of sustainability. The company also broadened their collaboration with US pharmaceutical company Humacyte, Inc., a specialist in developing and manufacturing universally implantable biotechnologically produced human tissue.
Despite these advancements, 2022 witnessed a decline in net income compared to 2021. In the company’s annual report, CEO Helen Giza attributed this downturn to the impacts of the COVID-19 pandemic, a challenging labor market, staffing shortages, high turnover rates and significant rate increases, all contributing to the decline in operating income and net income. To mitigate these costs, financial support was received from the US government. Although Fresenius Medical Care anticipates slow revenue growth for 2023, they expect a resurgence in net income growth by 2024.

6) Becton, Dickinson & Company
2022 Revenue: For the year ended September 30, 2022, Becton, Dickinson & Company’s (BD’s) annual revenue was $18.87 billion, a 1.36 percent decrease from $19.13 billion from the year ended September 30, 2021.
2022 Net Income: Becton, Dickinson & Company’s (BD’s) annual net income for the year ended September 30, 2022, was $1.78 billion, a 14.96 percent decrease from $2.09 billion for the year ended September 30, 2021.
2022 R&D Spend: For the year ended September 30, 2022, Becton, Dickinson & Company (BD) spent $1.26 billion on R&D, a 1.80 percent decrease from $1.28 billion spent during the year ended September 30, 2021.
Becton, Dickinson & Company (BD) reported revenues of $18.87 billion in 2022, marking a 1.4 percent decrease from the previous year. This dip was largely due to a diminished demand for COVID-19 diagnostic kits and injection devices; however, the firm observed robust demand for its core products, especially in the Medical segment’s Medication Delivery Solutions and Pharmaceutical Systems units, as well as in the Life Sciences segment’s Integrated Diagnostic Solutions unit. Moreover, BD advanced its innovation pipeline with the launch of 25 significant new products within the year.
Revenue growth in the Medication Delivery Solutions unit during 2022 was driven by robust global sales of catheters and vascular care products. This was particularly attributed to competitive gains from peripherally inserted intravenous catheters and flush products.
BD completed the acquisition of Parata Systems, an innovative provider of pharmacy automation solutions, on July 18, 2022. This acquisition contributed positively to revenue growth in the Medication Management Solutions unit.
BD also made strides in becoming the first company to integrate flow cytometry with fluorescence imaging and image-based decisioning, enabling the sorting of individual cells based on their visual details at an exceptionally high speed (15,000 cells per second). Additionally, BD acquired Cytognos, a privately held company specializing in flow cytometry solutions for blood cancer diagnosis, minimal residual disease (MRD) detection and immune monitoring research for blood diseases. This acquisition granted BD access to advanced assays licensed from the EuroFlow Consortium, an independent scientific network in hematology and immunology. This extended BD’s already established 12-year licensing collaboration with EuroFlow, bolstering its portfolio of assays.
Another notable development was the launch of the BD COR MX instrument, a fully automated, high-throughput infectious disease molecular diagnostics platform, following 510(k) clearance from the FDA.
Furthermore, 2022 witnessed multiple collaborations for BD with esteemed institutions such as Mayo Clinic, Labcorp and Accelerate Diagnostics, bolstering the industry outlook.
Finally, BD reached an important milestone with the launch of the BD Effivax Glass Prefillable Syringe, reinforcing the company’s standing as the leading supplier of prefillable syringes worldwide.

7) Philips Healthcare
2022 Revenue: Philips Healthcare’s annual revenue was €17.83 billion ($18.75 billion USD) in 2022, a 3.91 percent increase from €17.16 billion ($20.28 billion USD) in 2021.
2022 Net Income: In 2022, Philips Healthcare’s net income was a loss of €1.61 billion ($1.69 billion USD), a 148.30 percent decrease from €3.32 billion ($3.93 billion USD) in 2021.
2022 R&D Spend: Philips Healthcare spent €2.10 billion ($2.21 billion USD) on R&D in 2022, a 16.45 percent increase from €1.81 billion ($2.13 billion USD) spent on R&D in 2021.
Philips, a global health technology leader, faced considerable challenges in 2022. Issues related to execution, quality, supply and a complex operating model impacted its business and financial performance. The recall of Respironics Sleep Therapy devices and ventilators further marred the company’s reputation; however, with a change in leadership from Frans Van Houten to the new CEO, Roy Jakobs, the company is now focused on enhancing its performance.
Despite these setbacks, Philips initiated the year strongly in terms of innovation, launching the industry’s first comprehensive, at-home, 12-lead electrocardiogram (ECG) solution for use in decentralized clinical trials. This addition has enhanced their growing portfolio of remote monitoring solutions designed to reduce patient attrition by minimizing site visits during trial periods.
In March 2022, the Philips Capsule Surveillance solution received 510(k) market clearance from the FDA, enabling its use across American healthcare systems. This system can stream data from any connected medical device, analyze the data to generate actionable insights and alerts, while simultaneously sending timely notifications to caregivers.
Philips also gained 510(k) market clearance from the FDA for its MR 7700 3.0T MR system — an innovation providing enhanced imaging and clinical diagnostics — alongside its latest compact ultrasound system, the innovative 5000 Compact Series. The company received FDA clearance for its SmartSpeed MR acceleration software, which introduces AI data collection algorithms to Philips’ existing Compressed SENSE MR engine. This allows for higher image resolution with three times faster scan times and virtually no loss in image quality.
Further, Philips signed a multi-year strategic partnership with Prisma Health, South Carolina’s largest non-profit healthcare system. This collaboration aims to assist Prisma Health in achieving enterprise interoperability, standardizing patient monitoring and pushing the boundaries in enterprise imaging solutions, all to boost patient care and elevate clinical performance.
With a strong pipeline and several products ready for launch, Philips aims to improve its performance in 2023.

8) Roche Diagnostics
2022 Revenue: In 2022, Roche’s Diagnostics division made ₣17.80 billion CHF ($18.64 billion USD), a 0.24 percent decrease from ₣17.84 billion CHF ($19.52 billion USD) in 2021.
2022 Net Income: Roche’s Diagnostics division made a net income of ₣3.32 billion CHF ($3.48 billion USD) in 2022, which the company says was not a notable change from their net income of ₣3.32 billion CHF ($3.63 billion USD) in 2021.
2022 R&D Spend: In 2022, Roche’s Diagnostics division spent ₣1.96 billion CHF ($2.06 billion USD), a 8.69 percent increase from ₣1.81 billion CHF ($1.98 billion USD) that the company spent on R&D in 2021.
Despite a decline in demand for COVID-19 products, Roche Diagnostics’ CEO Severin Schwan noted in the company’s annual report, “We achieved satisfactory results in 2022, thanks to growth in routine testing that more than offset the sales decrease due to lessened demand for COVID-19-related products.”
In 2022, Roche Diagnostics launched seven new platforms, 24 new tests and nine new digital solutions. The company also announced a collaboration with Bristol Myers Squibb to promote personalized healthcare, primarily supporting the development and deployment of two assays and two new digital pathology algorithms for use in clinical trials.
Significantly, Roche received full compliance under the In-Vitro Diagnostic Medical Devices Regulation (IVDR) from the European Union. With IVDR now in full effect, Roche plans to re-certify all remaining products (subject to the availability of external infrastructure), maintain comprehensive oversight on third-party readiness and ensure post-market surveillance.
Roche further bolstered its portfolio by gaining FDA approval for a label expansion of the Ventana MMR RxDx panel and launching the next-generation Ventana DP 600 slide scanner to help anatomic pathology laboratories expedite the digitization of their pathology workflow.
In addition, the company received FDA approval for the Pathway anti-HER2 (4B5) Antibody assay, intended to detect HER2 protein in patients with breast cancer. Roche Diagnostics also launched the Anti-PRAME (EPR 20330) Rabbit Monoclonal Primary Antibody to detect PRAME protein expression to help differentiate between benign and malignant lesions in melanomas.
Moreover, the company received an FDA EUA for the cobas MPXV test, designed for the cobas 6800/8800 systems. This automated PCR test detects the MPXV in lesion swabs collected from individuals suspected of having an MPXV infection.

9) Stryker
2022 Revenue: Stryker’s annual revenue was $18.45 billion in 2022, a 7.84 percent increase from $17.12 billion in 2021.
2022 Net Income: In 2022, Stryker made a net income of $2.36 billion, an 18.25 percent increase from $1.99 billion in 2021.
2022 R&D Spend: Stryker spent $1.45 billion on R&D in 2022, a 17.73 percent increase from the $1.24 billion Stryker spent on R&D in 2021.
Stryker saw a remarkable year in 2022, registering an 18.3 percent rise in net earnings. This success is attributable to several product launches throughout the year. Notably, Stryker announced the first surgical use of the innovative Shoulder iD Primary Reversed Glenoid, the first scalable patient-matched implant available for shoulder arthroplasty.
Stryker also introduced the PROstep MICA SOLO Guide, a comprehensive guide acting as a ‘third hand’ for surgeons during minimally invasive bunion procedures. The Joint Replacement division launched the Insignia Hip Stem, designed to optimize patient fit and facilitate implantation during muscle-sparing approaches for total hip and hemiarthroplasty procedures.
Additional key product introductions from Stryker’s portfolio include the EasyFuse Dynamic Compression system for high-demand foot and ankle applications, Gamma4 Hip Fracture Nailing system and Citrefix, a suture anchor system designed for foot and ankle surgical procedures.
In 2022, Stryker also strengthened its medical division by acquiring Vocera Communications, a leading provider in the digital care coordination and communication sector. By integrating innovative products with digital capability, Stryker is leveraging insights to enhance clinical, operational and financial outcomes across the care continuum, embodying what Stryker terms as Advanced Digital Healthcare.
On the regulatory side, Stryker’s Q Guidance system, a sophisticated planning and intraoperative guidance system for spine applications, and the OptaBlate bone tumor ablation system received 510(k) clearance from the FDA.
Stryker also published the results of a multicenter, single-blinded, randomized controlled trial comparing Stryker’s InSpace implant with partial repair for the treatment of full-thickness massive rotator cuff tears in The Journal of Bone and Joint Surgery (JBJS).
Beyond its business operations, Stryker demonstrated its commitment to environmental sustainability by achieving a 20 percent carbon reduction across all its facilities — a goal initially set for 2024. As stated by Kevin Lobo, Stryker’s chair and CEO, in the company’s 2022 annual report, “We will continue to make progress on our strategic pillars of Customer Focus, Innovation, Globalization and Financial Performance.”

10) GE Healthcare
2022 Revenue: In 2022, GE Healthcare’s annual revenue was $18.34 billion, a 4.30 percent increase from $17.59 billion in 2021.
2022 Net Income: GE Healthcare’s net income in 2022 was $1.92 billion, a 14.73 percent decrease from $2.25 billion in 2021.
2022 R&D Spend: In 2022, GE Healthcare spent $1.03 billion on R&D, a 25.74 percent increase from the $0.82 billion GE Healthcare spent on R&D in 2021.
GE Healthcare reported robust revenue growth for 2022, marking a 4 percent increase driven primarily by its Imaging and Ultrasound sectors. A significant development for the company in November 2022 was the announcement of its separation from the GE Company and its transition into an independent corporation, now known as GE Healthcare. CEO Peter J. Arduini expressed the momentous nature of this transition in the company’s news release, stating, “Today is an incredibly exciting day for GE HealthCare as we become an independent company and start a new chapter as a standalone global leader in precision care.”
GE Healthcare entered into an agreement to acquire Caption Health, a strategic move aimed at expanding its ultrasound business. This acquisition will support new users through FDA-cleared, AI-powered image guidance. Additionally, they will also acquire IMACTIS, which will enhance their capabilities in interventional guidance.
GE Healthcare also announced partnerships within the radiation oncology space with leading innovators such as Accuray, Elekta and RaySearch. Furthermore, a collaboration with Medtronic was initiated to address the specific needs and growing demand for care at ASCs and OBLs.
GE Healthcare further enhanced its imaging portfolio by introducing the next-generation Definium 656 HD, its most advanced fixed X-ray system to date. It also received FDA 510(k) clearance for its AIR Recon DL for 3D and PROPELLER imaging sequences for next-level image quality in MRI. The company also launched SIGNA Experience, a platform that integrates four synergistic technologies to ensure the smoothest MRI scanning experience for the patient, technologist and physician.
In April 2022, it received FDA pre-market approval (PMA) for its End-tidal (Et) Control software for general anesthesia delivery on its Aisys CS2 Anesthesia Delivery system. This approval solidifies GE Healthcare’s unique position as the only manufacturer in the US approved to offer general anesthesia delivery with end-tidal concentration control. Moreover, the launch of Portrait Mobile, a wireless patient monitoring system, enables continuous monitoring to assist clinicians detect early patient deterioration.
To tap into the homecare segment and further precision health, GE Healthcare announced its investment in Pulsenmore, a startup specializing in homecare ultrasound solutions.
With a strong pipeline of new products in growing diagnostic, therapeutic and monitoring markets, GE Healthcare anticipates accretive revenue growth and margin expansion moving forward, as stated in its annual report.
To have your company featured on Xtalks, please email Vera Kovacevic, PhD, at: [email protected]












Join or login to leave a comment
JOIN LOGIN