The neurology drug market continues to be dynamic with novel treatments that cater to a broad range of neurological and psychiatric conditions. In 2024, several blockbuster neurology medications are set to lead the market, capturing the attention of healthcare professionals, investors and patients alike. This year, key players like Roche, AbbVie and Janssen have bolstered their offerings with therapies targeting multiple sclerosis (MS), migraines, schizophrenia and more, each leveraging unique mechanisms of action and clinical benefits that resonate with patient needs and treatment gaps.
In this blog, we highlight the top 20 best-selling neurology drugs to watch in 2024, based on 2023 sales statistics. We discuss each drug’s approved uses, sales performance and why they’ve become essential in the neurology space. From market exclusivity to superior efficacy, these drugs show the advancements and patient impact within neurology — an area increasingly focused on improving quality of life and long-term outcomes. Here’s a look at what makes these therapies stand out and the trends driving their market success in 2024.
Note: When it comes to companies that report in foreign currencies, the conversion to US dollars uses the average annual exchange rates reported by the US Federal Reserve.
Related: Top 30 Drugs to Watch in 2024: Insights from 2023 Sales Data
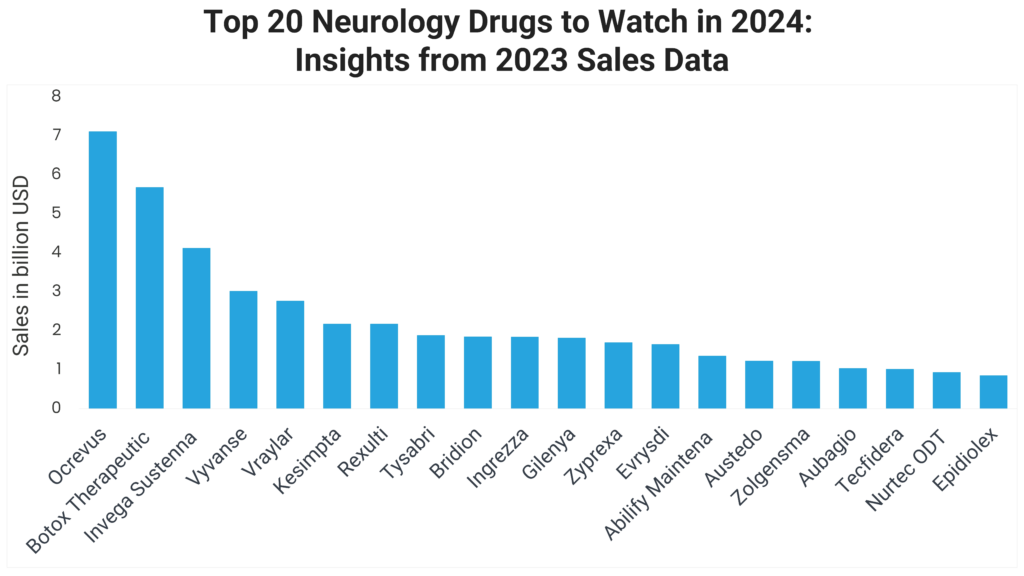
1. Ocrevus (Ocrelizumab)
Ocrevus 2023 sales: $7.103 billion
Company/developer: Roche
Date of first US Food and Drug Administration (FDA) approval: March 28, 2017
Indications Ocrevus is FDA-approved for: Ocrevus is a CD20-directed cytolytic antibody indicated for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease, in adults, as well as primary progressive multiple sclerosis (PPMS) in adults.
Price of Ocrevus: $78,858 annually
Why it sold so well: Ocrevus is a therapeutic monoclonal antibody that offers a unique scientific approach to treating MS. It specifically targets CD20-positive B cells, a type of immune cell that plays a crucial role in the progression of the disease. It is the first and only approved disease-modifying therapy for PPMS, a highly disabling form of MS, and is also approved to treat relapsing forms of MS (RMS).
In RMS, Ocrevus was shown to be superior to Rebif (interferon beta-1a), a commonly used treatment, over two years based on positive results from Phase III studies (OPERA I and OPERA II in RMS). Ocrevus was the first and only treatment to significantly slow progression over a median treatment duration of three years for PPMS in a clinical study.
According to Roche’s 2023 annual report, sales of Ocrevus were CHF 6.381 billion (around $7.1 billion USD), an increase of 13 percent, which included 11 percent growth in the US driven by both new and retained patients.
2. Botox Therapeutic (OnabotulinumtoxinA)
Botox 2023 sales: $5.673 billion
Company/developer: AbbVie
Date of first FDA approval: December 29, 1989
Indications Botox is FDA-approved for: Botox is an acetylcholine release inhibitor, and a neuromuscular blocking agent indicated for: chronic migraine; urinary incontinence and overactive bladder; cervical dystonia; neurogenic detrusor overactivity; spasticity; severe axillary hyperhidrosis; Botox cosmetic (for cosmetic purposes).
Price of Botox: Cost depends heavily on the condition and dose required, but the list price is $1,292 for a 200-unit vial.
Why it sold so well: Botox is FDA-approved for the treatment of chronic migraines and that is currently the only chronic condition for which it has received approval. It is a biologic drug available exclusively as a brand-name medication, with no generic or biosimilar forms available. Botox is one of the most prescribed branded treatments for chronic migraine prevention, offering rapid relief with just four treatments per year.
3. Invega Sustenna (Paliperidone Palmitate)
Invega Sustenna 2023 sales: $4.115 billion
Company/developer: Janssen Pharmaceuticals, Inc.
Date of first FDA approval: July 31, 2009
Indications Invega Sustenna is FDA-approved for: Invega Sustenna is indicated for the treatment of schizophrenia in adults, and schizoaffective disorder as monotherapy and as an adjunct to mood stabilizers or antidepressants in adults.
Price of Invega Sustenna: The cost of Invega Sustenna intramuscular suspension, extended release (39 mg/0.25 mL) is around $620 for a supply of 0.25 mL, depending on the pharmacy.
Why it sold so well: Invega Sustenna is a once-a-month long-acting schizophrenia medication designed to overcome the adherence challenges associated with daily oral antipsychotics.
Since the original approval of Invega Sustenna in 2006, Janssen has also gained approvals for longer-acting versions of the drug.
Janssen has achieved blockbuster sales from its Invega franchise over the years. According to Janssen’s full-year 2023 earnings report, its family of schizophrenia medications (Invega Sustenna/Xeplion/Invega Trinza/Trevicta) generated nearly $2.9 billion in the US and $4.1 billion worldwide last year.
4. Vyvanse (Lisdexamfetamine)
Vyvanse 2023 sales: $3.012 billion
Company/developer: Takeda
Date of first FDA approval: February 23, 2007
Indications Vyvanse is FDA-approved for: Vyvanse is a central nervous system (CNS) stimulant indicated for the treatment of attention deficit hyperactivity disorder (ADHD), and moderate to severe binge eating disorder (BED) in adults.
Price of Vyvanse: The cost of Vyvanse 10 mg oral capsule is around $1,371 for a supply of 100 capsules.
Why it sold so well: Vyvanse was one of Takeda’s top-selling drugs for over a decade, but since generics were approved in 2023, its sales have consistently dropped. Vyvanse lost several crucial patent protections in 2023. The patents covering adult indications expired in February 2023, followed by those for pediatric use in August 2023.
According to the Centers for Disease Control and Prevention (CDC), ADHD is among the most common neurodevelopmental disorder in kids, and millions of adults are estimated to have it as well. Therefore, ADHD medication Vyvanse has always been in high demand.
The drug also faced shortages in the last couple of years, which also affected its revenue, but similar shortages were faced by generics therefore favoring Vyvanse sales. According to Takeda’s full-year 2023 annual report for the fiscal year ended March 31, 2024, revenue from Vyvanse/Elvanse was 423.2 billion JPY (around $3.012 billion USD).
5. Vraylar (Cariprazine)
Vraylar 2023 sales: $2.759 billion
Company/developer: AbbVie
Date of first FDA approval: September 17, 2015
Indications Vraylar is FDA-approved for: Schizophrenia in adults; acute treatment of manic or mixed episodes associated with bipolar I disorder in adults; treatment of depressive episodes associated with bipolar I disorder (bipolar depression) in adults; adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD) in adults.
Price of Vraylar: The wholesale acquisition cost for a 30-day supply of Vraylar is $1,446.55 as of January 2024.
Why it sold so well: MDD is a leading cause of disability worldwide, and its large patient population makes it an attractive treatment market, especially as diagnoses have risen since the pandemic.
Vraylar is the first and only dopamine and serotonin partial agonist that is FDA-approved for the most common forms of depression. It gained approval for acute treatment of manic or mixed episodes associated with bipolar I disorder and treatment of schizophrenia in adults in 2015. It has won more FDA label expansions since then. In 2019, it gained approval for the treatment of depressive episodes associated with bipolar I disorder (bipolar depression) in adults, and in 2022, for the treatment of MDD in adults as an adjunct to antidepressants.
So far more than 1 million patients have been treated with Vraylar.
6. Kesimpta (Ofatumumab)
Kesimpta 2023 sales: $2.171 billion
Company/developer: Novartis Pharmaceuticals
Date of first FDA approval: August 20, 2020
Indications Kesimpta is FDA-approved for: Kesimpta is a CD20-directed cytolytic antibody designed for the treatment of relapsing forms of MS in adults, encompassing clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease.
Price of Kesimpta: The list price of Kesimpta is $8,736 per treatment.
Why it sold so well: Sales of Kesimpta surged in the third quarter of 2023, exceeding expectations by reaching $657 million. This increase was propelled by adjustments reflecting higher-than-anticipated prices in the European market.
Kesimpta stands out by providing robust efficacy alongside a favorable safety profile, and it offers the convenience of self-administration at home.
Studies have demonstrated reductions in the risk of relapses, confirmed disability progression, Gd+ T1 brain lesions and new/enlarging T2 lesions. A post hoc analysis indicates that Kesimpta has the potential to halt new disease activity in relapsing MS patients, with 47.0 percent and 87.8 percent of those treated with ofatumumab achieving no evidence of disease activity (NEDA-3) within the first year (0 to 12 months) and second year (12 to 24 months) of treatment, respectively.
7. Rexulti (Brexpiprazole)
Rexulti 2023 sales: $2.169 billion
Company/developer: Otsuka Pharmaceutical Co., Ltd and H. Lundbeck A/S
Date of first FDA approval: July 10, 2015
Indications Rexulti is FDA-approved for: Rexulti is classified as an atypical antipsychotic with multiple indications. It is recommended as an adjunctive therapy alongside antidepressants to treat MDD in adults. Additionally, it is prescribed for the treatment of schizophrenia in both adult and pediatric patients aged 13 years and older. Moreover, Rexulti is used for managing agitation linked to dementia resulting from Alzheimer’s disease.
Price of Rexulti: The list price of Rexulti is $1,419 per month.
Why it sold so well: According to Lundbeck’s 2023 annual report, revenue from Rexulti/Rxulti reached 4,525 million DKK (around $657 million USD) in 2023. Otsuka’s 2023 report showed that revenue from Rexulti/Rxulti was 212.509 billion JPY (around $1.513 billion USD). The notable growth in Rexulti sales is attributed to robust momentum, particularly in the US, Canada and Brazil.
In November 2023, the FDA issued supplementary approval for Rexulti oral tablets, specifically for addressing agitation linked to dementia caused by Alzheimer’s disease. Recent efficacy data from clinical studies reveals noteworthy and clinically meaningful enhancements in agitation symptoms, coupled with favorable tolerability.
8. Tysabri (Natalizumab)
Tysabri 2023 sales: $1.876 billion
Company/developer: Biogen
Date of first FDA approval: November 23, 2004
Indications Tysabri is FDA-approved for: Tysabri is indicated as monotherapy for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease, in adults.
Price of Tysabri: The cost for Tysabri (300 mg/15 mL) intravenous concentrate is around $8,999.72 for a supply of 15 mL. In Canada, Tysabri costs approximately $40,000 per year.
Why it sold so well: RMS is a progressive disease that damages the central nervous system. Although there is no cure for the disease, many relapsing MS treatment options are available that can help slow its progression. Tysabri is one of these drugs that can slow the disease progression. It has been proven to slow disability progression and reduce the number of relapses and new brain lesions. Tysabri works differently from other MS drugs by preventing the white blood cells of the immune system from entering the brain and spinal cord. It still remains one of the top revenue generators despite facing competition from biosimilars.
9. Bridion (Sugammadex)
Bridion 2023 sales: $1.842 billion
Company/developer: Merck
Date of first FDA approval: December 15, 2015
Indications Bridion is FDA-approved for: Bridion is indicated for the reversal of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide in adults and pediatric patients aged two years and older undergoing surgery.
Price of Bridion: The list price of Bridion is $1,296.60 for a box of ten 2 mL single-dose vials (100 mg/mL).
Why it sold so well: In 2015, Bridion received FDA approval for reversing neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. This injectable drug, a modified gamma cyclodextrin, works by diffusing through the plasma and capturing the blocking agents. This groundbreaking drug marked a significant advancement in anesthesia management, especially in reversing the effects of neuromuscular blockade. It provides numerous benefits, such as shorter recovery times, improved patient safety and enhanced control for medical professionals.
Bridion remains one of Merck’s top-performing assets and continues to demonstrate robust growth as it has revolutionized anesthesia management, establishing a new standard of care in the field. Bridion received extended patent protection in 2023, which shields it from generic competition until January 2026.
10. Ingrezza (Valbenazine)
Ingrezza 2023 sales: $1.836 billion
Company/Developer: Neurocrine Biosciences
Date of first FDA approval: April 11, 2017
Indications Ingrezza is FDA-approved for: Ingrezza is a vesicular monoamine transporter 2 (VMAT2) inhibitor indicated for the treatment of adults with tardive dyskinesia and chorea associated with Huntington’s disease.
Price of Ingrezza: The average wholesale price per Ingrezza capsule (40 mg) is $300.56.
Why it sold so well: In August 2023, the FDA further approved Ingrezza for the treatment of adults with chorea associated with Huntington’s disease. This approval is based on its demonstrated three-fold greater improvement in chorea severity compared to the placebo. Clinical results indicated that patients receiving valbenazine experienced a mean reduction (improvement) in Total Maximal Chorea (TMC) score of 4.6 units, compared to 1.4 units in the placebo group (p < 0.0001). Two weeks after discontinuation of the study medication, TMC scores for patients who had received valbenazine reverted to baseline.
11. Gilenya (Fingolimod)
Gilenya 2023 sales: $1.811 billion
Company/developer: Novartis
Date of first FDA approval: September 22, 2010
Indications Gilenya is FDA-approved for: Gilenya is used for the treatment of relapsing forms of MS, to include clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease, in patients ten years of age and older.
Price of Gilenya: The cost for a Gilenya oral capsule 0.5 mg is around $373 per capsule, or $11,204 for a supply of 30 capsules.
Why it sold so well: Gilenya was the first pill for RMS and has helped more than 310,000 patients worldwide. It represented a milestone for the treatment of MS, but now it has lost patent protection, and several generics are now available in the market. Gilenya was once one of the top revenue generators for Novartis but competition from generics has made a dent in its revenue.
12. Zyprexa (Olanzapine)
Zyprexa 2023 sales: $1.695 billion
Company/developer: Eli Lilly and Company developed the drug; on April 21, 2023, Cheplapharm announced that it has agreed with Eli Lilly on the acquisition of the worldwide commercial rights for Zyprexa.
Date of first FDA approval: September 30, 1996
Indications Zyprexa is FDA-approved for: Zyprexa is an atypical antipsychotic indicated for the treatment of schizophrenia in adults and adolescents ages 13 to 17; acute treatment of manic or mixed episodes associated with bipolar I disorder and maintenance treatment of bipolar I disorder in adults and adolescents ages 13 to 17; as medication therapy for pediatric patients with schizophrenia or bipolar I disorder only after a careful diagnostic evaluation and consideration of the potential risks; adjunct to valproate or lithium in the treatment of manic or mixed episodes associated with bipolar I disorder.
Price of Zyprexa: The cost of Zyprexa oral tablets (2.5 mg) is around $431.23 for a supply of 30 tablets.
Why it sold so well: In Eli Lilly’s 2023 annual report, revenue from Zyprexa, including the sale of the rights for the olanzapine portfolio, was $1,694.8 million.
In July 2023, Eli Lilly transferred the rights to its olanzapine portfolio, including Zyprexa, to the European company Cheplapharm Arzneimittel GmbH (Cheplapharm). Under the agreement, Lilly received an initial payment of $1.05 billion in cash and is set to receive an additional $305 million one year after the transaction’s closing.
13. Evrysdi (Risdiplam)
Evrysdi 2023 sales: $1.646 billion
Company/developer: Genentech (Roche)
Date of first FDA approval: August 7, 2020
Indications Evrysdi is FDA-approved for: Evrysdi, a survival of motor neuron 2 (SMN2) splicing modifier, is developed for the treatment of spinal muscular atrophy (SMA) in pediatric and adult patients.
Price of Evrysdi: The cost of Evrysdi is $340,000 annually.
Why it sold so well: Evrysdi is the first oral medication, and the second drug authorized for the treatment of SMA. In clinical trials, following 12 months of treatment, 33 percent of infants on Evrysdi achieved the significant milestone of independent sitting for at least five seconds — a noteworthy departure from the typical disease progression, as nearly all untreated infants with infantile-onset SMA struggle to sit independently. Moreover, after 23 or more months of treatment, a remarkable 40 percent of infants were able to sit without support for at least 30 seconds.
14. Abilify Maintena (Aripiprazole)
Abilify Maintena 2023 sales: $1.350 billion
Company/developer: Otsuka Pharmaceutical Co., Ltd. and H. Lundbeck A/S
Date of first FDA approval: February 28, 2013
Indications Abilify Maintena is FDA-approved for: Abilify Maintena (aripiprazole) is a prescription medication administered by injection under the supervision of a healthcare professional. It is indicated for the treatment of schizophrenia in adults and for maintenance treatment of bipolar I disorder in adults. This formulation provides an effective and convenient option for individuals dealing with these psychiatric conditions under the guidance of healthcare providers.
Price of Abilify Maintena: The list price for Abilify Maintena is $2,388.47.
Why it sold so well: Abilify Maintena is protected by multiple US patents covering various aspects, including specific formulations of the active ingredient, manufacturing processes, delivery devices, approved indications and methods of use. These patents have different expiration dates, with the latest set to expire in 2034.
In the Phase III clinical trial leading to its approval, Abilify Maintena significantly delayed the recurrence of mood episodes over a 52-week period compared to a placebo. The analysis showed a statistically significant extension in the time to relapse for the Abilify Maintena group versus the placebo (log-rank test p < 0.0001).
15. Austedo (Deutetrabenazine)
Austedo 2023 sales: $1.225 billion
Company/developer: Teva Pharmaceuticals USA, Inc.
Date of first FDA approval: April 3, 2017
Indications Austedo is FDA-approved for: Austedo is a VMAT2 inhibitor indicated for treating chorea associated with Huntington’s disease and tardive dyskinesia.
Price of Austedo: The list price of Austedo tablets (6 mg bottle/60 count) is $4,881.00.
Why it sold so well: Study findings revealed that patients undergoing treatment with Austedo exhibited a notable improvement of 4.4 units in maximal chorea scores from baseline to the maintenance period, as opposed to the 1.9 units observed in the placebo group at Week 12. Furthermore, the total maximum chorea scores of patients who had received Austedo returned to baseline levels at the Week 13 follow-up visit.
16. Zolgensma (Onasemnogene Abeparvovec-xioi)
Zolgensma 2023 sales: $1.214 billion
Company/developer: Novartis
Date of first FDA approval: May 24, 2019
Indications Zolgensma is FDA-approved for: Zolgensma is a gene therapy that uses an adeno-associated virus vector to treat pediatric patients under two years of age with SMA caused by bi-allelic mutations in the survival motor neuron 1 (SMN1) gene. This condition results from these mutations, which lead to inadequate SMN protein expression. Administered intravenously, Zolgensma enables cell transduction to restore SMN protein expression.
Price of Zolgensma: Zolgensma has a price of $2.1 million for a single-dose treatment.
Why it sold so well: Zolgensma is a gene therapy classified as personalized or precision medicine, as it is designed to address specific issues arising from an individual’s unique genetic code. Data from Zolgensma’s Phase III trial revealed prolonged event-free survival, improvements in motor function and significant milestone achievements in patients with SMA type 1.
In 2020, Novartis Gene Therapies launched an innovative early access program for Zolgensma, designed to facilitate prompt patient access. This program offers flexible options such as retroactive rebates, deferred payments, installment plans, outcome-based rebates and partnerships with healthcare systems to enhance disease management. Currently, there are over 40 early access and pay-for-performance agreements established in multiple markets globally. Zolgensma has received approval in 51 countries.
17. Aubagio (Teriflunomide)
Aubagio 2023 sales: $1.030 billion
Company/developer: Sanofi
Date of first FDA approval: September 12, 2012
Indications Aubagio is FDA-approved for: Used for treatment of relapsing forms of MS to include clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease, in adults.
Price of Aubagio: The cost of Aubagio 7 mg oral tablet is around $9,851 for a supply of 30 tablets.
Why it sold so well: Aubagio belongs to the selective immune-suppressants drug class and is a disease-modifying drug commonly used to manage the symptoms of MS. Despite being Sanofi’s blockbuster MS drug, Aubagio saw a significant decline in sales due to competition from generics that entered the market in mid-March 2023.
18. Tecfidera (Dimethyl Fumarate)
Tecfidera 2023 sales: $1.012 billion
Company/developer: Biogen Inc.
Date of first FDA approval: March 27, 2013
Indications Tecfidera is FDA-approved for: Used for the treatment of patients with relapsing forms of MS.
Price of Tecfidera: The price of a 14-capsule supply of Tecfidera 120 mg oral delayed-release capsules is approximately $2,330.
Why it sold so well: In Phase III clinical trials of Tecfidera capsules, a significant decrease in the proportion of individuals experiencing relapses at the two-year mark was observed compared to those on a placebo. Participants on the twice-daily dosage of Tecfidera exhibited a 49 percent reduction in the risk of relapse compared to the placebo group. Tecfidera also demonstrated a 44 percent reduction in the average annual number of relapses (annualized relapse rate) in comparison to the placebo. Furthermore, the two-year progression rate was 16 percent for twice-daily Tecfidera, contrasting with 27 percent for placebo — a noteworthy 38 percent reduction in the risk of disability progression.
According to Biogen’s 2023 annual report, worldwide revenue for Tecfidera fell by $431.4 million, dropping from $1,443.9 million in 2022 to $1,012.5 million in 2023, representing a 29.9 percent decline. This decrease was primarily due to reduced demand stemming from the introduction of several generic versions of Tecfidera in North America, Brazil and select European Union (EU) countries.
19. Nurtec ODT (Rimegepant)
Nurtec ODT 2023 sales: $928 million
Company/developer: Developed by Biohaven Pharmaceuticals, acquired by Pfizer in 2022
Date of first FDA approval: February 27, 2020
Indications Nurtec ODT is FDA-approved for: Used in adults for the acute treatment of migraine with or without aura and for the preventive treatment of episodic migraine.
Price of Nurtec ODT: The price of eight Nurtec ODT oral tablets, disintegrating (75 mg each) is around $1,061.46.
Why it sold so well: More than 6.7 million prescriptions for Nurtec ODT have been written by over 162,000 healthcare providers. In June 2023, Pfizer partnered with Lady Gaga — an Academy Award, Golden Globe and 13-time Grammy-winning actress and philanthropist — to promote Nurtec ODT in the US. According to IQVIA data, as of March 31, 2024, this oral calcitonin gene-related peptide (CGRP) receptor antagonist has been the top choice in its class, both in total prescriptions and in new prescriptions, since August 6, 2021.
20. Epidiolex (Cannabidiol)
Epidiolex 2023 sales: $845 million
Company/developer: Jazz Pharmaceuticals
Date of first FDA approval: June 25, 2018
Indications Epidiolex is FDA-approved for: Indicated for the treatment of seizures associated with Lennox-Gastaut syndrome (LGS), Dravet syndrome or tuberous sclerosis complex (TSC) in patients one year of age and older.
Price of Epidiolex: The price of Epidiolex is around $32,500 annually.
Why it sold so well: Epidiolex (Epidyolex outside the US) saw a 15 percent rise in net product sales in 2023, reaching $845.5 million. In the fourth quarter of 2023, sales grew 16 percent to $240.6 million compared to the same quarter in 2022. Epidyolex is approved in over 35 countries outside the US, with further launches and reimbursement expected by the end of 2024.










Join or login to leave a comment
JOIN LOGIN