Update (June 13, 2019): Today, Orchestra BioMed announced a strategic partnership with Tokyo-based Terumo Corporation, a leading medical device manufacturer, to develop and commercialize the Virtue SEB. Terms of the deal include an upfront payment of $30 million, plus milestone payments as the company advances clinical development and seeks global market approval. CEO of Terumo Medical Corporation North America said the company plans on making the non-coated drug-eluting angioplasty balloon its “flagship therapeutic product”.
“A key aspect of this strategic partnership is that Terumo has made a strong commitment to finance and execute a global clinical program in collaboration with Orchestra BioMed that aims to secure regulatory approvals in multiple large commercial markets such as the U.S., Japan and China,” said Orchestra Chairman and CEO, David Hochman, in an email to Xtalks. “Terumo has a proven global distribution infrastructure and expertise to make Virtue SEB broadly accessible to physicians and patients worldwide, pending regional approvals.”
Originally published on May 1, 2019:
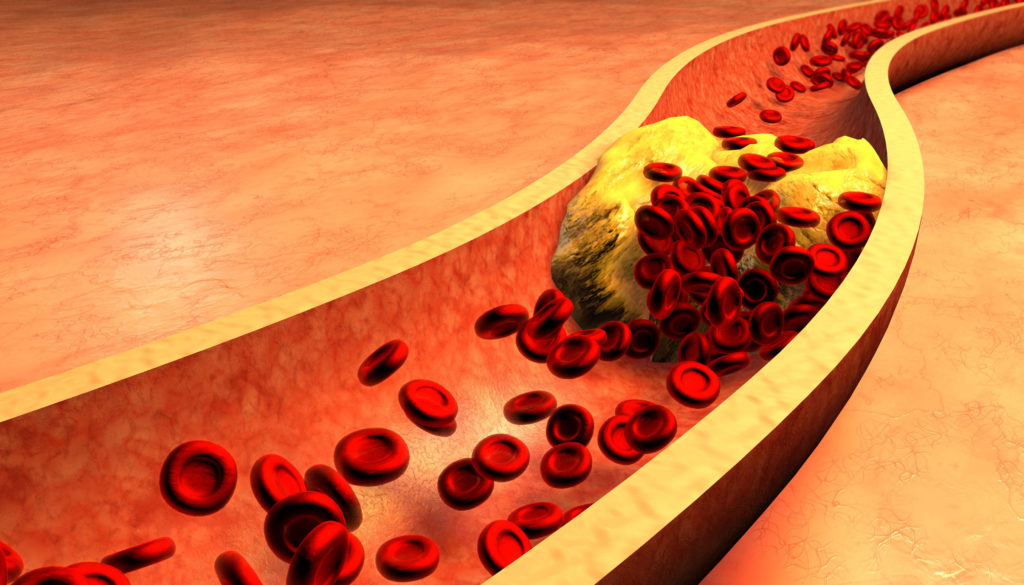
Coronary artery disease, caused by the buildup of plaque in blood vessel walls, is the most common form of heart disease in the US. To remove the blockage and support the damaged blood vessel, interventional cardiologists may place a permanent drug-eluting stent in the affected area or expand the lumen through balloon angioplasty.
While both strategies can help patients in the short-term, they are at risk of developing coronary in-stent restenosis (ISR), which occurs when the blood vessel becomes reclogged. ISR accounts for over 10 percent of interventional cardiology procedures each year according to the American College of Cardiology’s National Cardiovascular Data Registry, and places a significant burden on a patient’s quality of life.
The most common off-label solution for ISR treatment is placement of another drug-eluting stent. However, this approach has been associated with a long-term increased risk of reintervention, heart attack and death.
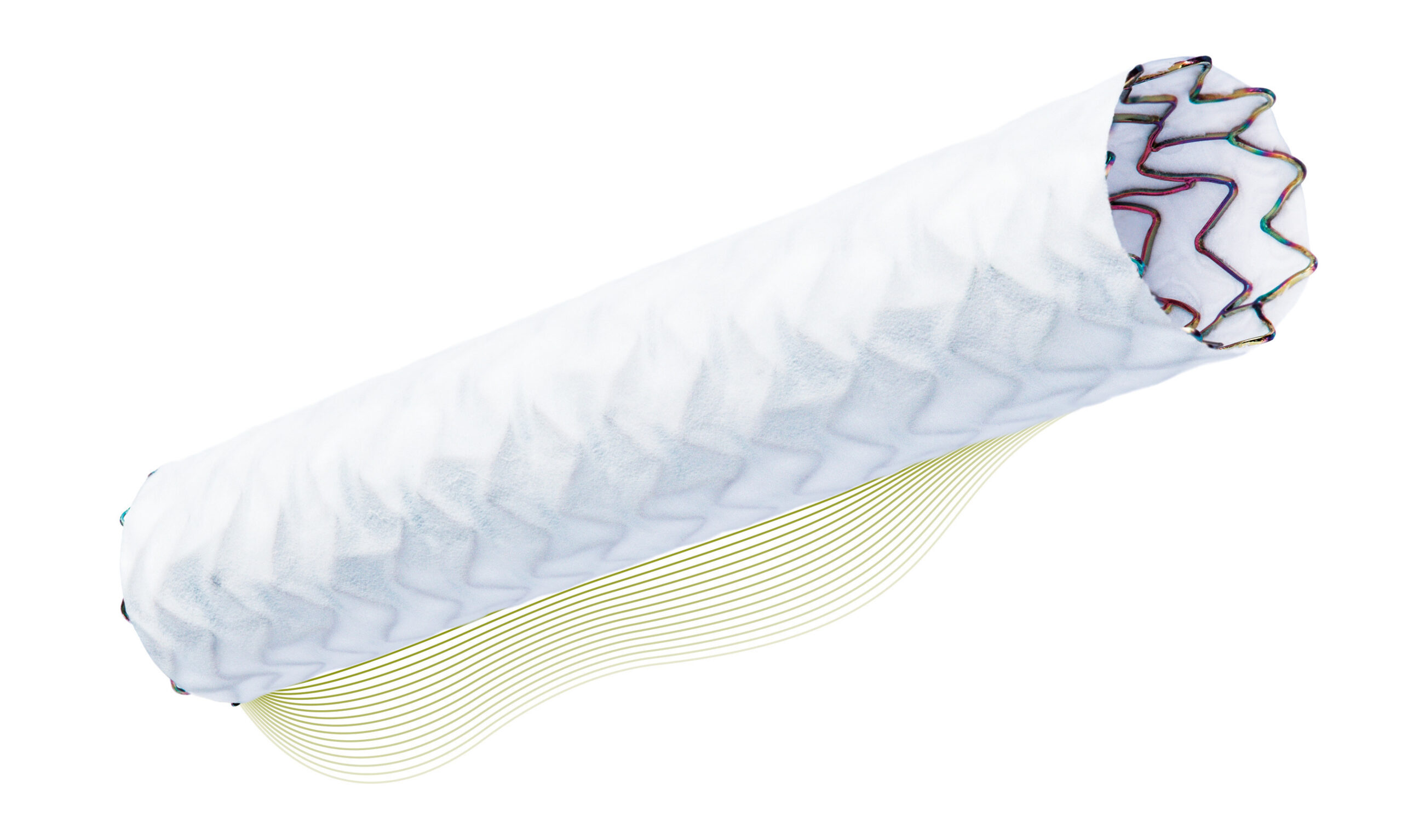
Orchestra BioMed, a biomedical innovation company, hopes to improve patient outcomes by using a medical device that combines the benefits of anti-restenotic pharmaceutical agents used on drug-eluting stents with the mechanical properties of balloon angioplasty without the need for a permanent stent implant. Their flagship product, Virtue® Sirolimus-Eluting Balloon (SEB), received Breakthrough Device Designation from the US Food and Drug Administration (FDA) last week to help turn this hope into a reality sooner.
“We plan to fully leverage the benefits of FDA Breakthrough Device designation as we seek to accelerate the US clinical and regulatory development of Virtue SEB with the goal of providing physicians and patients with the benefits of our novel therapeutic device,” said Darren R. Sherman, President, Chief Operating Officer and Founder of Orchestra BioMed in a press release.

The Virtue® SEB is a microporous balloon angioplasty device that delivers sirolimus, the gold standard drug for preventing restenosis, directly to the artery wall during balloon angioplasty. Unlike other drug-releasing devices on the market, Virtue® SEB is not coated.
“The concept of a drug-coated balloon is to transfer the drug through contact,” said David Hochman, Chairman, CEO and Founder of Orchestra BioMed in an interview with Xtalks. “But there’s a lot of concern over the loss of the drug during navigation to the lesion as well as dislodgment of large polymer coating particulates which can potentially block downstream vessels.”
The technology in Orchestra BioMeds’s drug-eluting balloon enables sustained release of sirolimus over 30 days without involving a permanent implant. Balloon angioplasty and drug transfer occur in the same 30-60 seconds typical of an interventional procedure using an angioplasty balloon, explained Hochman.
In a three-year clinical study, researchers showed that patients who received Virtue® SEB achieved a low rate of target vessel failure (5.6 percent), a critical safety endpoint which looks at both major adverse cardiac events and need for re-intervention. What’s more, none of the patients experienced any major complications during the procedure and over the acute follow-up period.
Currently, the only approved strategies for treating ISR in the US include plain old balloon angioplasty or intravascular radiation therapy, both of which have limitations in terms of safety and efficacy. The FDA Breakthrough Device Designation enables the company to accelerate product development, testing and priority of regulatory review. To Hochman, this designation also indicates that the FDA recognizes there is an unmet clinical need.
“We also see the recognition that our technology does represent a potential breakthrough that can offer an important improvement in patient care,” added Hochman. “The designation will allow us to have important, more frequent and thoughtful interactions with FDA as we look to advance the product towards approval.”
For now, the company looks forward to advancing clinical studies for Virtue® SEB. Hochman also sees potential uses of their proprietary bioabsorbable, polymer-encapsulated system in other therapeutic areas.








Join or login to leave a comment
JOIN LOGIN