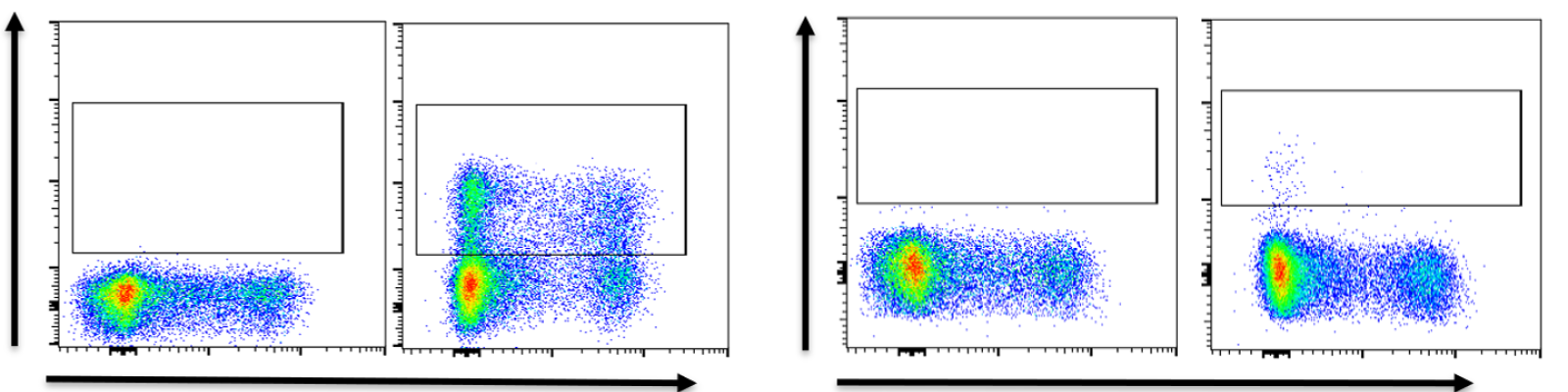
Assessing humoral immunogenicity is an interesting challenge for adeno-associated virus (AAV)-based gene therapies. While most people are concerned about how to deal with pre-existing antibodies and their impact on cutpoints — such as using total antibody or neutralizing antibodies for inclusion/exclusion, including/excluding based on these results, and a number of other concerns — often times just coming up with the right assay format for measuring these antibodies is overlooked.
The goal of this webinar is to provide case studies for the two typically used assay formats for measuring total antibodies (direct and bridging) and discuss the challenges and rationales for using each of them in the AAV immunogenicity space. The case studies will be followed by a panel discussion with an expert scientific team to take questions and discuss other possibilities for assay format selection.
Register for this webinar to learn about the rationale behind choosing an assay format for total antibody (TAb) assays for AAV-based gene therapies, including the pros and cons of each format.
Speakers

Jim McNally, Ph.D., Chief Scientific Officer, BioAgilytix (Moderator)
Dr. McNally has an extensive background in bioanalytical assay development and program leadership spanning nearly 20 years in the pharmaceutical and biotechnology industry.
Prior to joining BioAgilytix, Dr. McNally was Executive Director at CRISPR Therapeutics, where he led a team of scientists to develop a portfolio of assays to support development of gene-based therapeutic candidates throughout their lifecycle. He has also previously held roles at Genzyme, Pfizer, EMD Serono, and Shire which have given him broad experience in the development of large molecule, gene therapy, and cell therapy biotherapeutics.
Dr. McNally is a recognized thought leader in the development and application of bioanalytical methods used in regulatory submissions and is specifically skilled in progression of biotherapeutics from research through clinical development. He has a special interest in the immunogenicity of biotherapeutics and leads an industry-wide working group to address this issue. A key part of his role at BioAgilytix is advising on emerging scientific developments and providing scientific and regulatory guidance.
Dr. McNally earned his B.S. in Biology from Mississippi State University, his Ph.D. in Viral Immunology from Louisiana State University School of Medicine in Shreveport, and his Post-Doc in Viral Immunology from University of Massachusetts Medical School.

Jay Stringer, Ph.D., Manager I, BioAgilytix
Jay has more than 10 years’ combined experience working with numerous immunoassay formats in academic, clinical, and BioAgilytix labs. Jay earned his PhD in Pharmacology from Duke University. Before joining BioAgilytix, Jay worked in the UNC Hospital’s McLendon Clinical Labs performing large and small molecule CLIA regulated testing and at a startup designing assays for a novel combination flow cytometer and hematology analyzer.
At BioAgilytix, Jay currently manages a team of scientists and analysts developing, qualifying, and validating assays to support sample testing for clients. He has supported multiple clients in different species for AAV ADA testing utilizing a simple bridging approach on the MSD platform.

Luke Armstrong, Ph.D., Manager II, BioAgilytix
Luke has over a decade of experience in reagent product development and contract research in cell-based assays, pharmacology, molecular cloning, protein expression, and immunoassays. Before joining BioAgilytix in 2018, he worked as an Associate Director of Cell and Molecular Biology at Charles River Laboratories and ChanTest, and was Manager of Cell Biology R&D at EMD Millipore.
In his role at BioAgilytix, Luke manages a research and development and operations team providing quality services in cell-based assays for detection of neutralizing antibodies to large molecule biologic drugs. Luke earned his Ph.D. in Biochemistry and Molecular Biology from the University of California, Davis, and was a postdoctoral fellow in the Department of Biochemistry at the University of Washington.

Todd Lester, Associate Director, BioAgilytix
Todd is a seasoned biotechnology and pharmaceutical project management professional. In his role at BioAgilytix, he oversees and leads all technical aspects of complex bioanalytical studies, including design, interpretation, analysis, documentation, and reporting of results, investigations, and deviations.
Todd has broad GxP expertise and is well-versed in the regulations and filing requirements for FDA and EMA, particularly regarding immunogenicity assessment strategy and data interpretation. He holds a Bachelor of Science in Biological Sciences from Cornell University and is a licensed Project Management Professional (PMP) with the Project Management Institute (PMI).

Xiaoxi Yang, Ph.D., Manager I, BioAgilytix
Xiaoxi has 10+ years of experience in assay/device development research in the Pharmaceutical/Diagnostics fields. Xiaoxi earned her Ph.D. in Biomedical Engineering from Tulane University. Before joining BioAgilytix in 2018, she worked as a Product Development Lead on Companion Diagnostics at BioMedomics.
In her role at BioAgilytix, Xiaoxi manages an operations team to develop and validate ligand binding assays to support the trials for large molecule biologic drugs. She led and executed several anti-drug antibody (ADA) assays to support adeno-associated virus (AAV)-based gene therapy programs with varying formats.

Marianne Scheel Fjording, MsC, PhD., Scientific Officer Executive Director, BioAgilytix
Marianne has an extensive background in large molecule bioanalytical assay set-up and assay validation with 25+ years of experience working in the pharmaceutical and biotechnology industry. Marianne earned her Ph.D. in Biochemistry and Molecular Biology from the University of Copenhagen, Denmark
In her role as Scientific Officer, she provides scientific and regulatory support to clients and the operational teams at BioAgilytix as well as bioanalytical strategies to clients.
Who Should Attend?
- Bioanalytical Scientists
- Clinical Operations
- Regulatory Teams
What You Will Learn
Join this webinar to learn about:
- The thought process behind choosing an assay format for total antibody (TAb) assays for AAV-based gene therapies
- The pros and cons for each format
- Case studies for the two typically used assay formats
Xtalks Partner
BioAgilytix
BioAgilytix is a leading global contract research organization focused on supporting pharmaceutical and biotech partners in all phases of drug development. With laboratory locations in North Carolina’s Research Triangle Park; Cambridge, Massachusetts; San Diego, California; Melbourne, Australia and Hamburg, Germany, BioAgilytix provides PK, immunogenicity, biomarkers, and cell-based assay services supporting the development and release testing of therapeutics across a number of industries and disease states.
BioAgilytix offers assay development, validation, and sample analysis under non-GLP, GLP, and GCP, as well as GMP quality control testing (i.e., product release testing, stability testing, etc.). BioAgilytix also offers diagnostic testing services at its CLIA-certified, CAP-accredited Boston laboratory.
BioAgilytix’s team of highly experienced scientific and QA professionals ensures high-quality science, data integrity and regulatory compliance through all phases of clinical development. BioAgilytix is a trusted partner to many top global pharmaceutical and biotech companies.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account