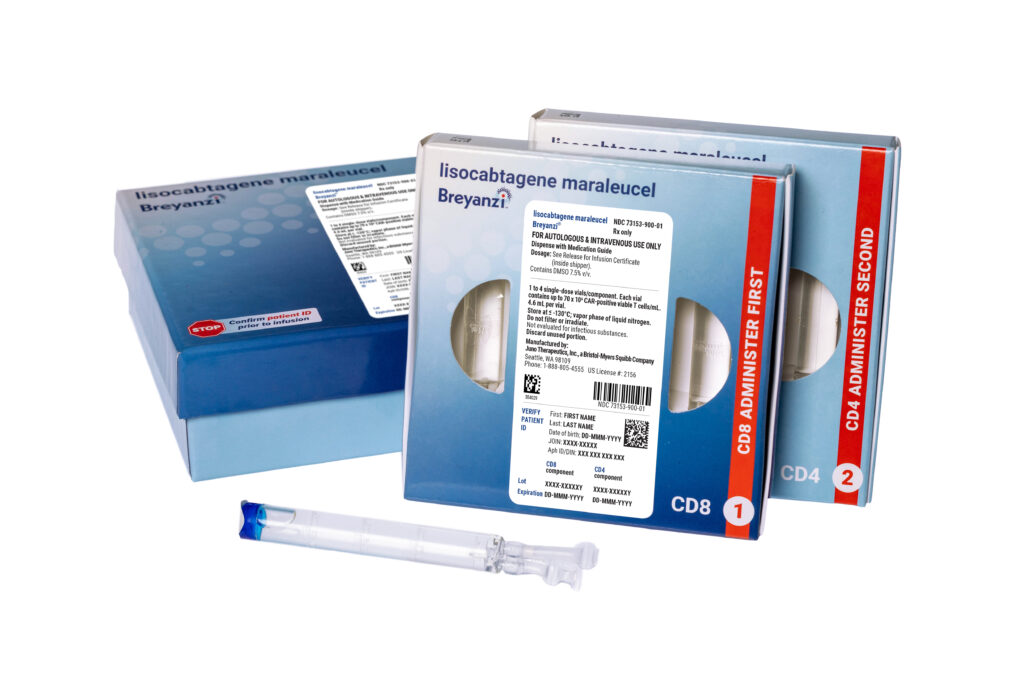
The FDA has announced the approval of Breyanzi (lisocabtagene maraleucel) as the first CAR T-cell therapy in the US for adults with marginal zone lymphoma (MZL).
The single infusion CD-19 targeting CAR T-cell therapy is now authorized for use in adults with relapsed or refractory MZL who have previously received at least two lines of systemic therapy.
With the approval, Breyanzi has also become the first CAR T-cell therapy approved for five different types of blood cancer, edging out Gilead’s CD-19-targeting blockbuster Yescarta and Tecartus, which have a combined four approvals in the space. Tecartus is the only one in the class that is approved for B-cell acute lymphoblastic leukemia.
First approved in 2021 for large B-cell lymphoma, Bristol Myers Squibb’s Breyanzi is nearing blockbuster status, with a 58% increase in revenues to $359 million in Q3 and $907 million in total sales so far this year.
The CAR T-cell therapy is also now approved to treat mantle cell lymphoma (MCL) and follicular lymphoma (FL), as well as chronic lymphocytic lymphoma (CLL) and small lymphocytic lymphoma (SLL).
Gilead’s Yescarta hit $1.6 billion in sales in 2024, while Tecartus had more modest earnings of $403 million.
MZL is a rare, slow-growing cancer of the lymphatic system. It accounts for roughly 7% of all B-cell non-Hodgkin lymphomas, with about 7,460 new cases diagnosed annually in the US, according to the FDA.
Patients whose disease stops responding to or returns after standard treatment face a poorer outlook.
As a CD-19-directed CAR T-cell therapy, Breyanzi works by genetically engineering a patient’s own T cells to recognize and latch onto the CD-19 protein on cancerous B cells to kill them.
The treatment involves collecting the patient’s T cells through leukapheresis, modifying them in a lab and then infusing them back into the patient after a short course of preparatory (lymphodepleting) chemotherapy.
The FDA’s approval was based on data from the MZL cohort of the Phase II Transcend FL study. In the trial, among the 66 patients who received the full treatment, the overall response rate (ORR) was 95.5% (partial or complete response), with 62.1% achieving a complete response.
In the follow-up period, ~90% of responders remained in remission at two years.
XTALKS WEBINAR: Clinical Trial Success through Novel Clinical In-Use Strategies
Live and On-Demand: Thursday, January 22, 2026, at 10am EST (4pm CET/EU-Central)
Register for this free webinar to learn how clinical in-use studies contribute to clinical trial success by strengthening the safety, efficacy and reliability of biotherapeutic drug administration.
“Patients living with marginal zone lymphoma, a subtype of indolent non-Hodgkin lymphoma, generally see success with initial therapy, but a subset of patients ultimately experience multiple relapses over the course of many years, creating a pressing need for new treatment options with durable outcomes,” said M. Lia Palomba, MD, Transcend FL study investigator and lymphoma and cell therapy specialist at the Memorial Sloan Kettering Cancer Center, in a news release from BMS.
“The FDA approval of liso-cel in relapsed or refractory marginal zone lymphoma is a significant advancement in redefining the treatment landscape and providing patients with an option that has demonstrated high rates of responses with an established safety profile.”
As with other CAR T-cell therapies, Breyanzi carries risks, with the most commonly reported side effects, including cytokine release syndrome (CRS), which occurred in 76% patients in the trial, with 4.5% having grade ≥3 CRS. Other side effects included headache, tremor and encephalopathy, with each occurring in 21% of patients.
Given the typically indolent but relapsing nature of MZL, where patients often undergo multiple rounds of therapy over many years, these results are especially meaningful.
The approval expands the reach of CAR T-cell therapy beyond aggressive or more common lymphomas to include a rarer, indolent subtype, offering a new treatment option for patients with limited alternatives.
Breyanzi now stands out as the CD19-directed CAR T-cell therapy with the broadest indication range among B-cell malignancies, according to Lynelle B. Hoch, president, Cell Therapy Organization, Bristol Myers Squibb.
“This approval in a fifth cancer type reflects our bold vision to bring the transformational potential of cell therapy to more patients,” said Hoch. “Breyanzi is the first and only CAR T cell therapy approved for this patient population, demonstrating Bristol Myers Squibb’s deep commitment to expanding access and reaching as many patients as possible with this innovative, practice-changing treatment.”
BMS obtained Breyanzi through its 2019 acquisition of Celgene for $74 billion. Celgene had acquired the CAR T-cell therapy less than a year before via its $9 billion purchase of Juno Therapeutics.











Join or login to leave a comment
JOIN LOGIN