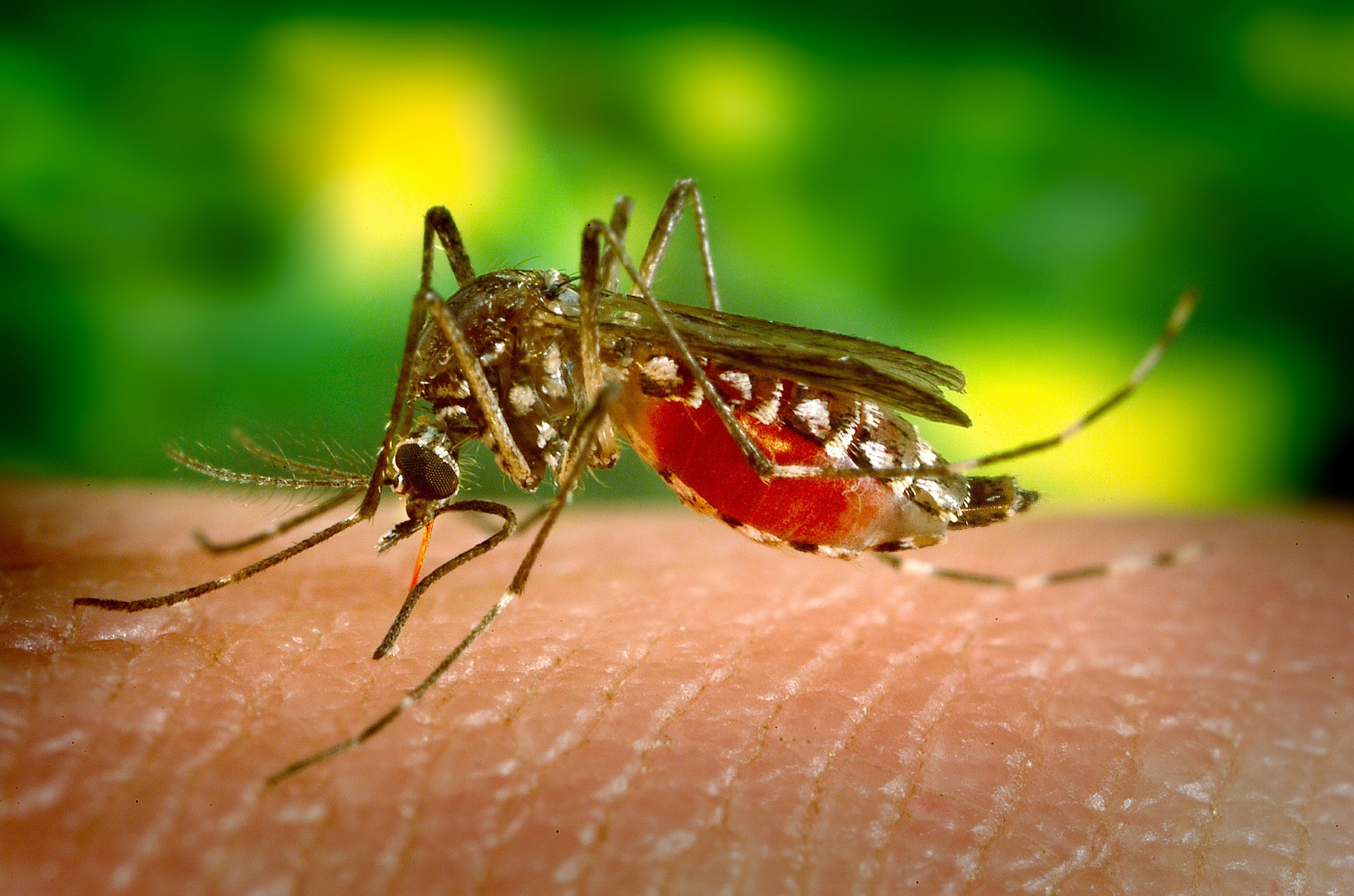
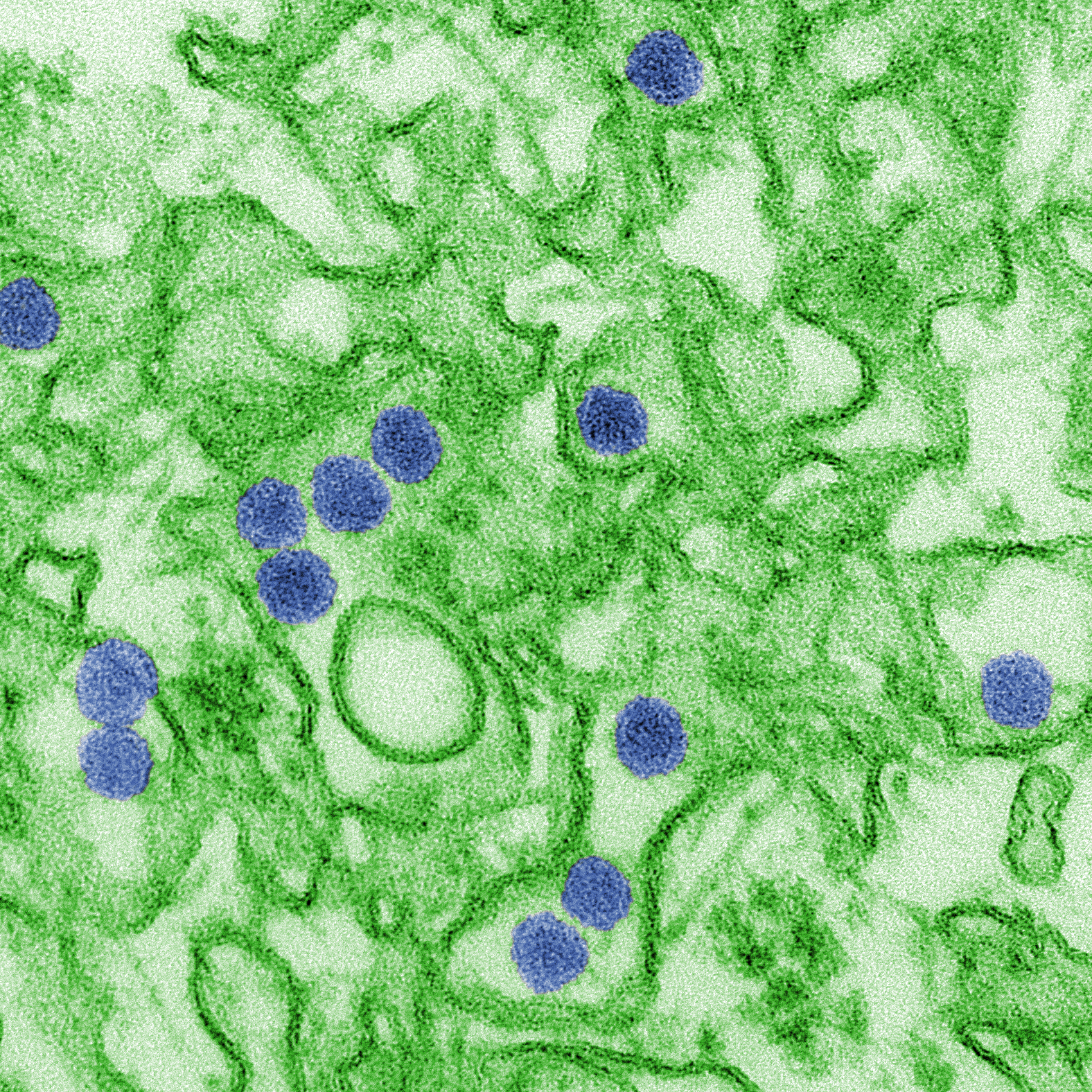
Recently, the news around infectious diseases have been dismal at best: rising cases of measles in the US, a growing shortage of Ebola vaccines in the Congo and increasing antimicrobial resistance in the UK. But last week, some good news: the US Food and Drug Administration authorized the marketing of the first diagnostic assay for Zika virus — the insidious mosquito-borne infection.
The last major outbreak of Zika in 2015-2016 took doctors and scientists by surprise. Flu-like symptoms may manifest between 3-14 days post-infection, but most people do not present with symptoms at all. Pregnant women infected with Zika are at risk of stillbirth or preterm birth and their babies may be born with abnormally small heads (microcephaly) or other congenital abnormalities. Since the outbreak, researchers and global health agencies have been working to better understand the disease and develop the appropriate prevention, diagnostic and treatment tools to manage it.
To date, 19 Zika Virus diagnostic tests have received Emergency Use Authorization (EUA) from the FDA, a designation reserved for unapproved interventions for serious or life-threatening diseases when no adequate alternatives are available. Now, one of those EUA tests is approved for market use.
The ZIKV Detect 2.0 IgM Capture ELISA, developed by InBios International, diagnoses patients with Zika virus by looking for Zika-specific antibodies in the blood. IgM antibodies tend to emerge early in the immune response, therefore, this assay could potentially detect Zika early and enable quicker access to treatment.
Over 800 test samples and multiple studies were reviewed to reach this approval decision. The test is intended to be used as an adjunct to clinical observation, patient history and other types of laboratory evidence.
“Today’s marketing authorization is a great demonstration of the FDA’s work to protect the public health in emergency response situations,” said Acting FDA Commissioner Dr. Ned Sharpless in a statement. “We ensured there were tests made available quickly under EUA, but we continued to work with diagnostic manufacturers to take the next step of ensuring products were FDA reviewed for safety and effectiveness and authorized under our traditional premarket authorities.”
This approval means subsequent Zika diagnostics companies can submit their tests for approval under the 510(k) premarket approval pathway. Companies might want to make note of this approval date especially if the FDA goes through with capping off the age of devices used as reference products.
For now, the 18 other Zika Virus EUA assays remain available for emergency use. This includes Abbott’s RealTime Zika test which looks for the virus in whole blood in a quick 6-hour turnaround.
Meanwhile, other biotech companies continue to explore quick, non-invasive diagnostic tests suitable for use in resource-limited areas. Such is the basis of a collaboration agreement between Group K Diagnostics and the Centers for Disease Control and Prevention (CDC), signed in March 2019. The biotech company hopes to design and test an assay that can detect Zika virus RNA in areas where traditional RNA detection instruments are unavailable.
Since the start of this year, only one case of Zika virus disease has been reported in the US by the CDC. The agency continues to advise travellers about how to protect themselves from Zika when travelling abroad. With the new diagnostic test approved, it’s possible that new cases can be caught and treated early.




Join or login to leave a comment
JOIN LOGIN