Advancements in science and technology have helped researchers develop new treatments for some of the most common diseases known to man. Diseases that were once considered death sentences are now chronic conditions, and some can even be cured.
For rare diseases, however, patients have limited treatment options. Out of the more than 7,000 known rare diseases, less than five percent have a US Food and Drug Administration (FDA)-approved therapy on the market. Unique research, clinical and regulatory challenges slow the drug development pipeline, leaving millions of rare disease patients with grim prognoses. Here, advanced therapies have the potential for the greatest impact.
Gene therapies aim to correct or replace dysfunctional genes that are the cause of many diseases. Approximately 85 percent of rare diseases are caused by a genetic mutation, making them ideal targets for gene therapy. Key to bringing gene therapies to market is a comprehensive understanding of clinical trial design and execution, and the regulatory landscape surrounding rare diseases.
To learn more about advanced therapy medicine products, clinical trial operations and regulations concerning rare diseases, watch this on-demand webinar from Medpace, a global clinical contract research organization.
Gene Therapy Success Stories
In the clinic, gene therapies are often administered using a viral vector, the most common being an adeno-associated virus (AAV). To administer this gene therapy, a healthy sequence of DNA is incorporated into the vector then the vector is injected into a patient. From there, the vector infects the patient’s cells just as a typical virus does, depositing the DNA sequence into the cell’s nucleus. This form of gene therapy, also called gene transduction, effectively causes “infected” cells to produce functional protein.
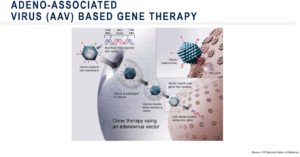
Viral vectors are mainly selected on their tropism, i.e. the preferentially targeting of specific organ and cell types, such as hepatocytes in case of liver directed gene therapy. Also immunogenicity is an important factor for choice of AAV serotype. Infection and transduction rates can be diminished naturally occurring neutralizing antibodies against the virus. Adeno-associated viral vector 5 (AAV5) for example has a low prevalence of neutralizing antibodies, possibly due to being divergent in the AAV phylogenetic tree.
Gene therapies have been investigated in a wide range of indications. For Dr. Marco Tangelder, senior medical director at Medpace, the most exciting advancements in gene therapy research are happening around hemophilia and ocular diseases.
“The innovation and R&D increase over the last few years is clear when we look at ocular diseases,” he says. Between 2016 and 2019 the number of gene therapy clinical trials for ocular diseases rose over 200 percent.
One of the greatest success stories in rare ocular diseases is the development of a gene therapy for the treatment of biallelic RPE65 mutation-associated retinal dystrophy. The RPE65 gene encodes for the RPE65 protein which is involved in vitamin A metabolism and the conversion of retinol in the vision cycle. Mutations in this gene can lead to functional loss or even death of retinal pigmented epithelial cells, resulting in early vision loss and blindness. In total, approximately 1,000-2,000 people in the US are affected by this form of hereditary retinal dystrophy.
Spark Therapeutics developed LuxturnaTM, a gene therapy containing a wild-type copy of the RPE65 gene delivered in an AAV vector. Their pivotal phase III clinical trial provided sufficient efficacy data to earn them FDA approval for the treatment of RPE65 mutation-associated retinal dystrophy in 2017.
Patients with the hereditary blood disorder, hemophilia B, have also seen significant progress made in gene therapy treatments. Individuals with hemophilia B either lack or possess a non-functional clotting factor IX (FIX) that may lead to spontaneous bleeding. Managing severe disease involves expensive frequent prophylactic infusions of recombinant FIX, thus a huge burden for patients and society.
Several gene therapies including AMT-060 and 061 and SPK-9001 deliver functional wild type copies or mutated variants of FIX using AAV vectors. Trial data shows recipients of such gene therapies no longer experience spontaneous bleeding or require FIX infusions, and even some patients achieve near-normal levels of FIX.
“These results are, in my view, a breakthrough in the treatment of hemophilia, as treatment can be considered even curative in some patients as they now have FIX levels within the normal range without any need for FIX prophylaxis and [are not experiencing] spontaneous bleedings,” says Dr. Tangelder.
Behind these success stories are years of R&D, designing and executing clinical trials, conducting long-term follow-up visits and working with regulatory authorities to bring these drugs to market. Clinical trials require significant organization, time and money to complete, but are critical in the drug development process.
Optimizing Clinical Trials For Gene Therapies
Rare disease trials come with their own unique set of challenges – limited knowledge of disease etiology, few or non-validated biomarkers or clinical outcome measures, few patients with high geographical dispersion and a limited number of experienced clinical investigators. Evaluating a gene therapy further complicates trial operations, as trial sponsors must cope with a lengthy feasibility process to ensure potential trial sites have the capacity and expertise to run a gene therapy trial.
Because rare diseases are so uncommon, researchers must rely on the natural history of disease to better understand disease progression. Using this strategy, they can derive outcomes assessments or endpoints that can be achieved by the therapy being tested. However, due to the low prevalence of these diseases, it is difficult to validate these assessments. For example, the ocular mobility test used in the LuxturnaTM trials had to be validated within the researchers’ clinical development program. In other rare disease trials, appropriate endpoints might not be known at all.
While six to eight percent of the world’s population is affected by a rare disease, rare disease patient recruitment and retention remains problematic. Trial sponsors must select trial sites that are geographically convenient for their target population and are adequately prepared to evaluate gene therapies. Sometimes, multiple trial sites need to be set up to meet these requirements.
“There could be two categories of sites: the sites that have gene therapy experience and the sites that do not have gene therapy experience but have potential subjects,” says Dr. Madhavi Malladi, clinical trial manager at Medpace. “In this situation, a centralized dosing approach can be adopted to include sites that do not have gene therapy experience. This strategy will help to meet recruitment goals and at the same time minimize patient burden.”
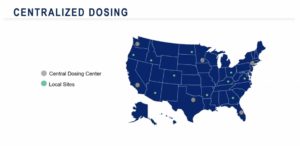
Sites without prior gene therapy experience can screen eligible patients before sending them to central dosing sites which have prior gene therapy experience. Once patients receive the intervention at the central dosing site, they can visit their local site (who do not have prior gene therapy experience) for future follow-up appointments. Trial sponsors should also be mindful of making travel accommodations for patients and their families to further reduce patient burden.
Coordinating between different trial sites requires impeccable communication prior to and throughout the trial. Dr. Malladi emphasizes that communication should be initiated early to delegate tasks, transfer medical records, arrange visit schedules and manage the clinical supply chain.
To further boost patient recruitment and retention, trial sponsors can lean on advocacy groups, ad campaigns and digital health technologies to increase awareness of ongoing trials. Healthcare providers and genetic counselors can also help dispel patient concerns over gene therapy safety, which is a major barrier to enrollment. While some patients might be skeptical of gene therapy trials with limited preclinical evidence, others – especially parents of children with rare diseases – are motivated to try a new therapy that could lead to a better life.
The complexity of rare disease and gene therapy trials might deter pharmaceutical companies from pursuing this research at all. However, the urgency to find new treatments for rare diseases has driven regulators worldwide to implement special guidelines or incentives to drive orphan disease research.
Regulatory Outlook for Rare Disease Treatments
The US, EU and Japanese regulatory bodies have the most comprehensive orphan drug programs in the world. Each country offers a specific, expedited regulatory pathway for orphan drugs as well as additional incentives to encourage and accelerate therapeutic development to address these unmet medical needs. Researchers can benefit from tax breaks, market exclusivity, substantial research grants and waived or reduced application fees.
However, not all orphan drug programs are the same. In fact, even the definition of a rare disease is not the same around the world.
“There is no harmonized global definition for rare disease, so qualifying criteria for one disease might be different in different countries,” says Dr. Todd Banks, director of regulatory affairs and regulatory intelligence at Medpace.
For instance, the EU will classify a condition as a rare disease if the prevalence is less than five people per 10,000 whereas Japan classifies rare disease based on a prevalence of less than 50,000 affected people.
Furthermore, companies must go through many regulatory hurdles to realize those financial incentives. One of their first tasks is to obtain an Orphan Drug Designation (ODD) in order to work under rare disease-specific guidelines. In 2017, the success rate of qualifying for US ODD designation was nearly 90 percent, owing to years spent refining guidelines, clarifying incentives and improving science and technology. However, only about 16 percent of the designated applications became successful commercial opportunities. If this figure seems low, consider that the success rate nearly two decades ago was less than eight percent.
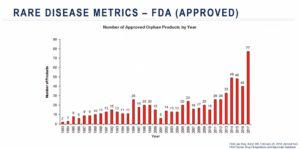
Relative to the number of known rare disease, only a small number of these diseases have approved treatments on the market. Scientific advancements and recent breakthroughs in the ophthalmology and hematology fields indicate that innovations are underway. Advocacy groups and regulatory authorities continue to encourage rare disease research, and more and more scientists are stepping up to the task. Finally, advances in gene therapies have opened the doors to potential therapeutic options for numerous rare diseases that were previously limited on available treatment options.
Dr. Todd Banks, Dr. Marco Tangelder and Dr. Madhavi Malladi share more insights on how advanced therapies are changing the landscape of rare disease in this on-demand webinar.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN