The EpiPen shortage in the US and Canada has been affecting patients for eight months, with no end to the manufacturing issues in sight. However, just two weeks after confirming that its EpiPen rival, the AUVI-Q, is widely available in the US, Virginia-based pharmaceutical company Kaléo has announced they’ll be selling their device in Canada as of September 7.
“As a company founded by patients with life-threatening allergies, we understand how critically important it is to be able to access an epinephrine auto-injector when it is needed,” said Spencer Williamson, President and CEO of Kaléo. “When Health Canada contacted us regarding our ability to help address the ongoing shortage of epinephrine auto-injectors, our team immediately went to work on a response plan.”
The move is the result of an Interim Order, signed by the Canadian Minister of Health Ginette Petitpas Taylor, which will allow AUVI-Q auto-injectors to be imported from the US. In working with a distributor, along with the provinces themselves, Kaléo is aiming to distribute the devices in a fair manner across Canada.
“I’m pleased that we have been able to secure a supply of epinephrine auto-injectors for Canadians with life-threatening allergies and their loved ones, particularly as families across the country are currently preparing for the start of the school year,” said Taylor. “We will continue to work with partners and stakeholders on long-term solutions to make sure life-saving auto-injectors remain available.”
In addition, Kaléo will be looking to get a similar device, Allerject, back onto the Canadian market. This auto-injector device was previously available in the country.
RELATED: Kaléo Reaffirms Availability of EpiPen Rival While Mylan Struggles with Manufacturing Issues
“Having a second supplier in the market is vital for Canadians with life-threatening allergies, including allergies to food, not only in this interim period of limited supply, but in the longer-term as a permanent solution,” said Jennifer Gerdts, Executive Director of Food Allergy Canada. “With AUVI-Q in the market, Canadian families get access to a different option for an epinephrine auto-injector. This is a significant step towards alleviating the pressure Canadian families living with life-threatening allergies, including allergies to food, are facing, and is a measure Food Allergy Canada has been advocating for.”
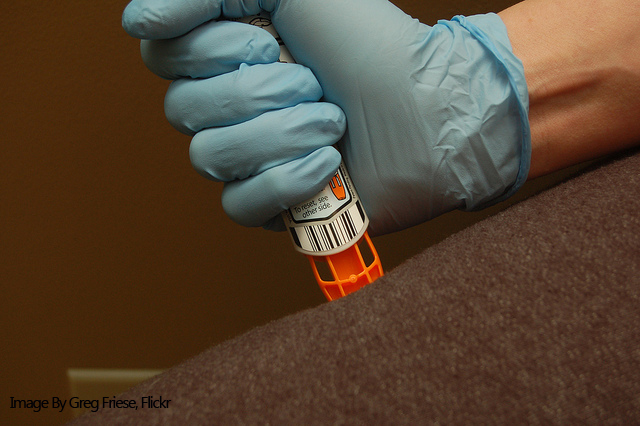
Like Mylan’s EpiPen, the AUVI-Q is an epinephrine-filled auto-injector device used in emergencies to treat severe allergies and anaphylaxis. However, the AUVI-Q is unique in that it features a retractable needle which is drawn back into the device after the active ingredient has been injected. In addition, it has voice instructions which help the user safely administer the treatment.
“We are taking the extraordinary measure to ship AUVI-Q auto-injectors approved for use in the US market to Canada to do our part during this immediate crisis,” said Phil Rackliffe, General Manager of Allergy and Pediatrics, at Kaléo. “In addition, we look forward to working with Health Canada to bring back Allerject in 2019 as an option for patients in need.”
While the availability of the AUVI-Q in Canada will help many patients get access to the potentially life-saving epinephrine shot, it likely still won’t be enough to ensure all patients are able to fill their prescription. Health Canada is urging pharmacists to carefully manage their supply of the AUVI-Q. In addition, both Canadian and US regulators have extended the expiration date of various lots of the EpiPen to prevent individuals with a working device from prematurely replacing them.
Mylan’s monopoly-like share of the North American auto-injector market is likely slipping in light of its persistent supply issues. In addition to the AUVI-Q device, Teva’s generic EpiPen has just been cleared by the FDA as the first non-branded version of the auto-injector to be sold in the country.







Join or login to leave a comment
JOIN LOGIN