Eli Lilly launched a 75-second TV commercial called “Change” in November 2024 to promote the company’s GLP-1 obesity drug Zepbound (tirzepatide).
The Zepbound TV commercial highlights the transformative journey of weight loss, encouraging viewers to embrace change and consider Zepbound as a catalyst for achieving their health goals.
The ad focuses on the theme of change to resonate with individuals seeking a meaningful and lasting impact on their health.
In the ad, individuals talked about how they “felt stuck” when it came to weight loss. But “Zepbound means change,” people in the ad say.
The spot depicts scenes of people enjoying various activities such as walking their dog, getting together with family for a meal and running a marathon.
Related: Eli Lilly’s Zepbound Approved as First Treatment for Obstructive Sleep Apnea
The commercial describes Zepbound’s mechanism of action as a dual receptor against that works by “activating two naturally occurring hormone receptors in [the] body [GLP-1 and GIP-1].”
Its dual action means that it “works differently,” according to the ad.
One man in the spot says that Zepbound is “changing what I believe is possible when it comes to weight loss.”
“It’s changing how much weight I lose, up to 48 lbs,” he says. This translates to an average weight loss of 21%, which was demonstrated in clinical trials (over a period of 72 weeks) that supported Zepbound’s FDA approval.
Zepbound can change “what happens,” for people struggling with weight according to the ad. “And when it comes to weight loss, change is good,” it ends off saying.
Zepbound was approved in 2023 as the obesity counterpart of Eli Lilly’s GLP-1 blockbuster Mounjaro. It rivals Novo Nordisk’s GLP-1 injection semaglutide, which is marketed as Wegovy for obesity and weight loss, and as Ozempic for diabetes.
Recent data from a head-to-head trial has shown that Zepbound leads to more substantial weight loss compared to Wegovy, with participants losing 47% more weight than those who took Wegovy.
This month, Zepbound won FDA approval for obstructive sleep apnea (OSA), making it the first approved drug for the condition.
Given the wild popularity of GLP-1 drugs, both tirzepatide and semaglutide products were on the FDA’s drug shortages list for the past couple of years. In October 2024, the agency announced that the shortage of tirzepatide had been resolved with Zepbound and Mounjaro making it back on pharmacy shelves.
Late last year, Zepbound brought in $1.26 billion in the third quarter and $1.9 billion in the fourth quarter, which were lower than both company and analyst expectations. Lilly chalked up the less-than-stellar sales performance to challenges in ramping up supply to meet soaring demand, coupled with uncertainties about the long-term market, highlighted by data showing that most users discontinue GLP-1 obesity drugs within a year.
If you want your company to be featured on Xtalks.com, please email [email protected].

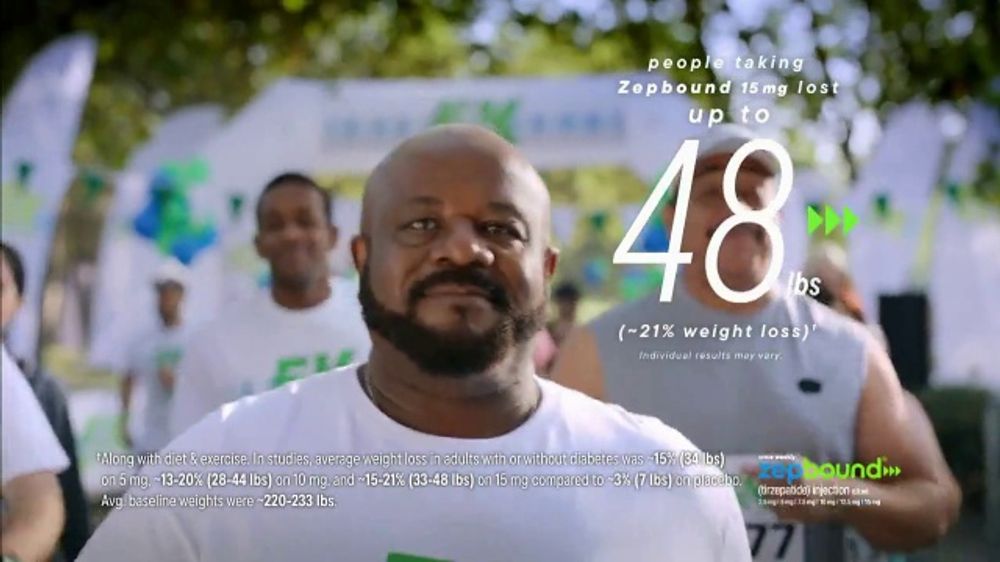










Join or login to leave a comment
JOIN LOGIN