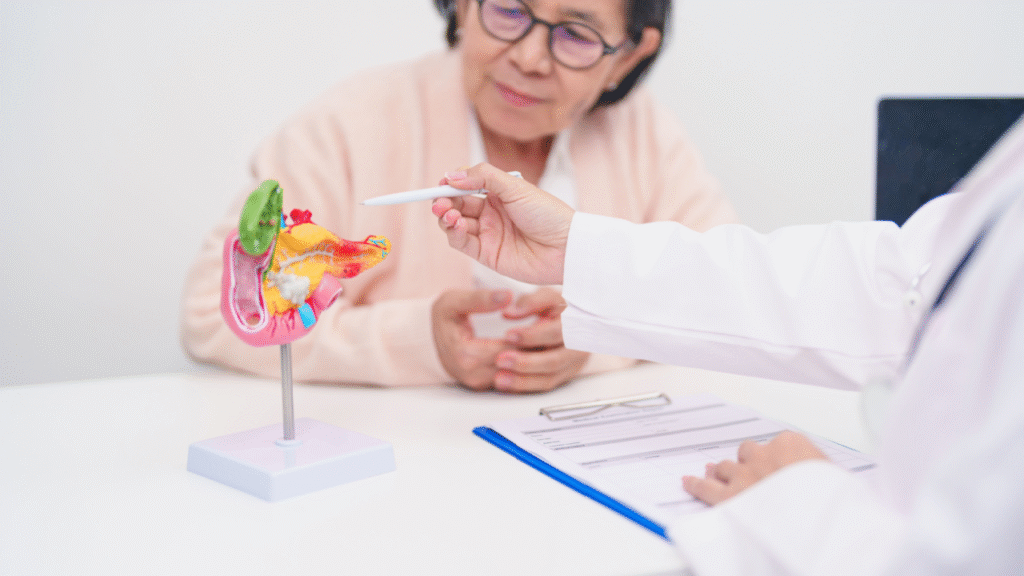
The FDA has approved Novocure’s Optune Pax, a portable medical device for adults with locally advanced pancreatic cancer. The device is meant to be used with standard chemotherapy.
The clearance brings a non-invasive treatment option to the disease.
For patients with locally advanced disease that is not surgically resectable, chemotherapy with or without radiation remains the standard approach. The approval of Optune Pax adds a biophysical treatment option that can be layered onto existing drug therapies without introducing additional systemic toxicity.
How Optune Pax Works
Optune Pax is a wearable device that delivers tumor treating fields (TTFields). TTFields are low-intensity, alternating electric fields applied to the abdomen through adhesive transducer arrays (sets of insulated electrode patches placed on the skin to deliver the electric fields). They interfere with cancer cell division during mitosis.
This mechanism allows the therapy to be combined with systemic treatments (which travel through the blood to treat the “whole” body) without adding systemic drug exposure.
Design and Use of the Wearable Device
The device is designed for routine use. Patients carry a small electric field generator in a shoulder or backpack, which lets them administer the therapy at home.
Patients are trained on device use, including array placement, battery management and replacement of the arrays several times per week.
The FDA reviewed Optune Pax through the premarket approval (PMA) pathway, the agency’s most rigorous regulatory process for medical devices. The device also has Breakthrough Device designation.
The agency described Optune Pax as the first device of its kind cleared to treat locally advanced pancreatic cancer.
Thursday, March 12, 2026, at 11am EDT (4pm CET/EU-Central)
Clinical Evidence Supporting the Approval
Approval was based primarily on results from the Phase III PANOVA-3 clinical trial, conducted under an Investigational Device Exemption.
Participants received either standard first-line chemotherapy with gemcitabine and nab-paclitaxel alone or the same regimen combined with TTFields delivered by Optune Pax. Patients were followed for a minimum of 18 months, with longer-term outcomes tracked for up to five years.
The trial met its primary endpoint of improved overall survival. In the intent-to-treat population, which includes all patients as originally assigned, those treated with Optune Pax plus chemotherapy achieved a median overall survival of 16.2 months, compared with 14.2 months with chemotherapy alone. This represented a statistically significant two-month improvement.
A modified per-protocol analysis, focused on patients who remained on therapy longer, showed a larger survival difference between treatment groups.
Several secondary outcomes also favored the Optune Pax group, including higher one-year survival rates and a longer time to pain progression. Patient-reported measures suggested slower deterioration in global health status, pain and certain digestive symptoms.
At the same time, the study did not demonstrate statistically significant differences between treatment arms in progression-free survival, objective response rate, local progression-free survival or tumor resectability. Taken together, these findings suggest that the observed benefit was primarily reflected in overall survival and symptom-related outcomes.
Safety findings were consistent with prior experience using TTFields in oncology.
New Developments in Pancreatic Cancer Research
While the Optune Pax brings a novel device-based approach, several pharmacological and biologic strategies are also being explored.
Valar Labs recently published a study validating an AI-based diagnostic that analyzes standard pathology slides to help predict which first-line chemotherapy regimen may be most effective for patients with advanced pancreatic cancer.
In drug development, the FDA has granted Orphan Drug designations to Novita Pharmaceuticals’ NP-G2-044, a small-molecule fascin inhibitor, and Arbele’s ARB1002, an ADC targeting the CDH17 protein, both of which are in clinical development.
On the imaging side, SOFIE Biosciences dosed the first patient in a Phase III clinical trial evaluating the PET radiotracer [¹⁸F]FAPI-74 for detecting metastatic disease in pancreatic ductal adenocarcinoma.
If you want your company to be featured on Xtalks.com, please email [email protected].












Join or login to leave a comment
JOIN LOGIN