A clinical research organization (CRO) is a company that delivers outsourced services to plan, manage and execute clinical trials for biotechnology, pharmaceutical, and medical device companies.
Medpace is a top-10, full-service CRO providing comprehensive Phase I to Phase IV clinical development services to support global research and the development of life-saving therapeutics and devices. While other CROs have grown primarily through mergers and acquisitions, Medpace has expanded through disciplined organic growth — expanding globally to operations in 40 countries and employing nearly 5,000 talented professionals. As a result, a culture of quality has been preserved with consistency in leadership and deep institutional experience.
Read on to learn why life science and clinical research professionals are choosing a career with Medpace.
Why Should One Consider Working for a CRO?
According to a report by ReportLinker, the pharmaceutical outsourcing market worldwide for discovery, preclinical and clinical services is estimated to be up to $70 billion in 2020 which is around 40 percent of the overall R&D spending by pharma. Out of that $70 billion, clinical-related services account for 40 to 60 percent.
As there is more disease burden in the growing population, there is an increase in demand for new treatments and more techniques for diagnosis. This has led to a sharp increase in the number of clinical trials (Figure 1).
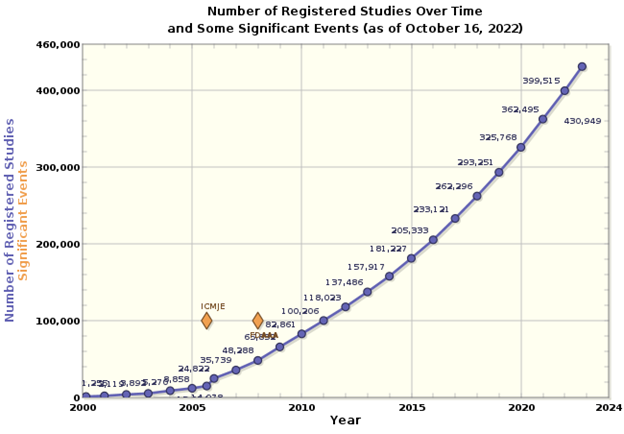
Figure 1. The number of registered studies from 2000 to 2022 on the ClinicalTrials.gov website. Source: https://ClinicalTrials.gov.
The reason why pharmaceutical, biotech, and medical device sponsors are spending resources on clinical-related services is that CROs are extremely experienced in conducting clinical trials. Particularly for up-and-coming biotechs that typically have limited resources, a full-service CRO like Medpace provides an all-in-one vendor approach that allows the Sponsors to outsource all aspects of the trial. This is in contrast to functional outsourcing models that outsource specific services like data management or trial monitoring.
Medpace believes the full-service model provides greater value to Sponsors: “When we can fully engage with our medical, regulatory and operational teams and work under our SOPs, we can perform at the highest levels to deliver quality results in the most timely and efficient manner. Competence and empowerment to coordinate all services under one roof provide an accountable, seamless, integrated and efficient platform – increasing quality and speed while significantly reducing a Sponsor’s need for duplicate management oversight.”
The pace at a CRO is fast with the opportunity for creative thinking and problem-solving. Fueling job satisfaction and fulfillment, CROs offer their employees the opportunity to work on clinical trials that are advancing medical therapeutics that could impact the lives of millions of people living with diseases. In addition, there are often exciting global opportunities and fast-track career ladders.
Why Work for Medpace?
Medpace’s mission is to “accelerate the global development of safe and effective medical therapeutics” and this CRO is committed to clinical research at the highest level of ethical standards.
This year marks the 30th anniversary of Medpace’s founding and the company is celebrating three decades of helping advance effective and safe medical treatments. The CRO is grateful to its employees who are making a difference through their commitment to improving the lives of patients, families, and communities by supporting the development of new and improved treatments.
In 2017, Medpace surpassed 2,500 employees globally. In 2022, Medpace has nearly 5000 employees across 41 countries. A company that achieves such growth in only five years clearly values its employees and knows how to nurture and retain them. Medpace provides its employees with the training and resources they need to succeed in their work and provides a wide array of internal growth opportunities.
Being a full-service CRO, Medpace offers the entire range of integrated services to execute even the most challenging global studies. Prospective employees should know that if they join Medpace, they will be part of an organization that has an overall goal to support the development of innovative drugs and devices.
Employees who join Medpace will be part of a talented team who is dedicated to their mission and has the expertise to complete challenging projects. The skills of these employees and the accomplishment of their work are reflected in Medpace’s multiple industry awards for outstanding performance, such as the 2022 CRO Leadership Awards for Capabilities, Compatibility, Expertise, Quality and Reliability. The industry awards are based on feedback from sponsors of drugs, biologics, and medical devices as well as clinical research sites.
What are the Biggest Benefits of Working for Medpace?
1) Working Today to Save Lives Tomorrow
Medpace’s values are “to create a unique culture where employees are part of a bigger purpose: to make a difference in the lives of millions today, tomorrow and for years to come. Together, we improve lives.” The work Medpace does provides hope for those living with debilitating diseases.
2) A Global Impact
Employees at Medpace know that their work contributes to making a positive change in people’s lives around the world and they understand how their role fits with the CRO’s overall mission. The drug and device development sectors are driven by people who are committed to improving and saving the lives of people around the world.
The fast globalization of many biopharmaceutical and medical device companies as well as international clinical trials have contributed to Medpace’s global reach. Medpace conducts clinical studies of all sizes across the world and has operations on six continents. Medpace employees that become part of their operational, regulatory, and medical teams will work beside experts that have country-specific knowledge and an awareness of the importance of local processes, culture, and language.
3) A Great Workplace
One of the benefits of working with Medpace is that their global reach provides job opportunities around the world. Medpace has offices throughout North America, Latin America, Asia, Europe, South Africa, and Australia. Most of their US employees are at their global headquarters in Cincinnati, Ohio, and there are also regional offices in Denver and Dallas.
Some of Medpace’s job postings require local, regional or international travel. There are also work-from-home options and a flexible work schedule when permitted.
Many of Medpace’s office locations have campus walking paths, on-site fitness centers, free and covered parking, discounts for local businesses, and an on-site marketplace. There are also company-sponsored social and wellness events.
Medpace is the recipient of numerous awards such as being recognized by Forbes as one of America’s Best Mid-Sized Companies in 2022and 2021; CRO Leadership Awards from Life Science Leader magazine; Top Cincinnati Workplace by the Cincinnati Enquirer; and was ranked 10 on the 2021 LinkedIn Top Companies list in Cincinnati.
4) Numerous Opportunities at Medpace
Since Medpace is a full-service CRO, the company offers different types of positions to fill their comprehensive range of services which include:
- Clinical monitoring
- Clinical trial management
- Medical leadership across numerous therapeutic areas and specialties
- Regulatory affairs and medical writing
- Biometrics and data sciences
- Drug safety and pharmacovigilance
- Quality assurance
- Clinical study management technology
Being a scientifically-driven and therapeutically aligned CRO, candidates can put their life-science backgrounds to work in a broad range of positions across a range of therapeutic and specialty areas. New hires will be able to work with Medpace’s exceptionally trained and experienced staff who have helped conduct global clinical trials in these areas:
- Autoimmune diseases
- Cardiovascular
- Endocrine and metabolic
- Gastroenterology
- Hematology and oncology
- Infectious diseases and vaccines
- NASH and liver disease
- Neurology and psychiatry
- Nephrology
- Ophthalmology
- Pediatrics
- Rare diseases
- Cellular and gene therapy
- Radiation therapy
Employees at Medpace have the chance to work with numerous sponsors and bring many drugs and devices to the market.
5) Entry-Level to Senior Positions
Full-service CROs cover a broad range of activities for the clinical trial process and that is why many CROs offer a broad range of jobs. There may also be career opportunities for different education and experience levels.
Medpace offers career opportunities for different education and experience levels, from entry-level to seasoned veterans of the CRO industry. Employees may also move from careers in academia and research, or clinical settings and apply their perspectives and backgrounds to clinical trials. Explore all of the career opportunities on the Medpace career website.
A Spotlight on Clinical Research Associates (CRAs)
The entry-level CRA position can be an excellent entry into clinical research, offering exceptional training and advancement opportunities. The position includes the chance to travel extensively to work with the sites where the trials are conducted. New CRAs benefit from Medpace’s industry-leading PACE training program (more on that below.)
Medpace has various opportunities for nurses and other healthcare professionals. The job titles for these professionals include nurse practitioner/clinical nurse specialist, clinical nurse, clinical nurse coordinator, clinical safety coordinator, regulatory submissions coordinator, clinical data reviewer, and clinical trial manager.
New PhDs with degrees in the life sciences often struggle to find their first rewarding job due to their high education level and little to no industry-related working experience. Medpace offers a range of opportunities for PhDs to use their expertise and many opportunities for PhDs do not require any previous experience. PhDs are encouraged to apply to positions that are aligned with their respective area. Examples of job titles for people with a Ph.D. include associate clinical trial manager, entry-level CRA, clinical data coordinator, and medical writer.
6) Training and Career Development Opportunities
Training is critical to an employee’s success at any CRO.
Medpace has earned a reputation for training and development because it is an innovative CRO that is up to date with the newest technologies and processes needed for its Phase I to IV clinical development services.
For instance, Medpace has a CRA Training Program called PACE® (Professionals Achieving CRA Excellence). Each CRA gets hands-on training in this training program detailing their job functions and all aspects of clinical research for drug, biologic, and medical device clinical trials. The PACE Training Program includes:
- US Food and Drug Administration (FDA) regulations
- European Union (EU) regulations
- Good Clinical Practices (GCP) as described by the International Conference on Harmonization (ICH)
- Industry best practices
- Therapeutic area reviews
- Medpace standard operating procedures (SOPs)
- Specialized departmental trainings
After completing this training program, Medpace CRAs have ongoing training in the form of monthly newsletters, quarterly seminars, and more.
Regarding career advancement, Medpace employees can build experience quickly because they work in an organization with ample resources and various projects that have international exposure. This allows Medpace employees to progress in their careers and apply to higher positions in the company. Employees who have proven their capabilities can have opportunities to advance to management roles. There are also growth opportunities across different functional areas at Medpace.
Qualities of Employees Who Excel at Medpace
CROs have an exciting and dynamic environment that may not be suitable for everyone. To excel at Medpace, a potential employee should have:
- Exceptional organizational skills
- The ability to multitask while prioritizing responsibilities
- Time management skills to adhere to a sponsor’s deadlines repeatedly
- The ability to communicate well with others verbally and in writing
- An attitude to relish challenging work
- A willingness to travel locally, nationally and internationally (only required for some positions)
Working at a CRO like Medpace will help professionals build a rewarding career working at a fast-growing organization with a mission that matters. The employees there feel a sense of purpose in their everyday lives as they work to provide hope for people across the world who are living with devastating diseases.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.






Join or login to leave a comment
JOIN LOGIN