In this webinar, the expert speakers will review strategies for phase-appropriate assessment of Fc-mediated functional activity and present new developments for complex modalities.
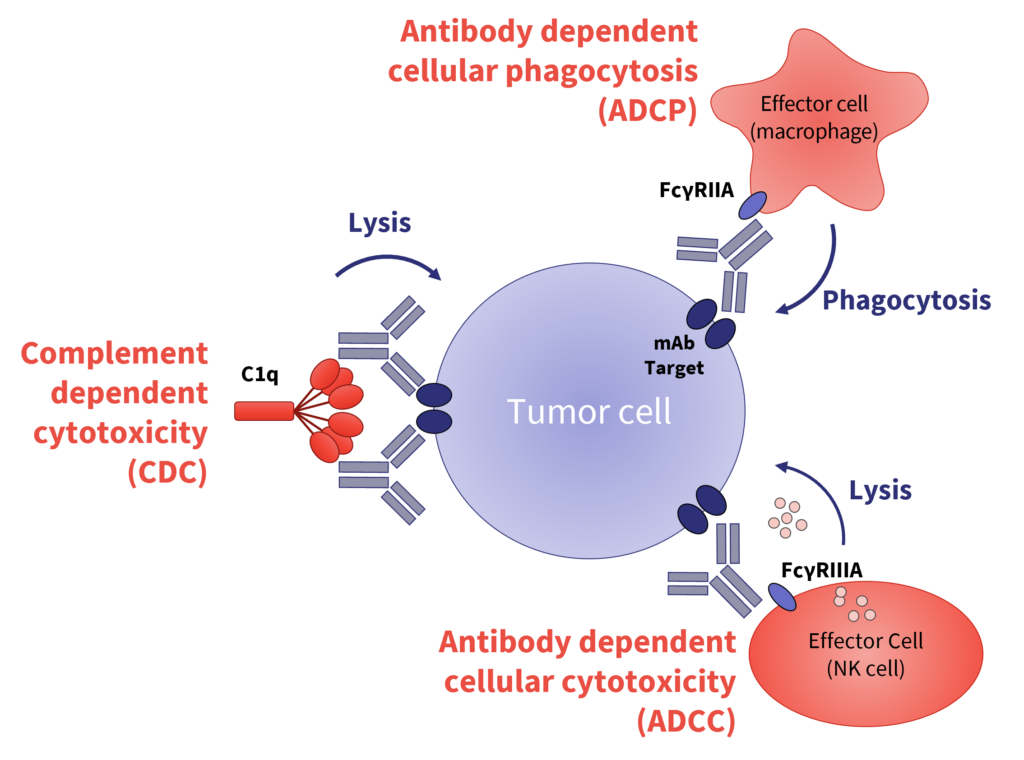
The success of many cancer therapeutics lies in their ability to induce cytotoxicity by NK cells (antibody-dependent cellular cytotoxicity (ADCC)), phagocytosis (antibody-dependent cellular phagocytosis (ADCP)) and complement-dependent cytotoxicity (CDC) against specific targets; therefore, appropriate assessment is crucial before progressing a drug into the clinic.
Importantly, demonstrating the lack of unwanted effector functions is required for safety purposes. During this webinar, the expert speakers will consider the key aspects for developing successful Fc-mediated functional assays and will review the benefits and challenges of a panel of assay formats for different purposes.
They will also present options for choosing the most suitable target cell lines, effector cells and appropriate controls. Moreover, they will showcase studies on how rigorous data trending leads to reliable assays and how more complex formats, such as how real-time spheroid cytotoxicity assays contribute to understanding the mode of action and selecting the most promising lead.
Register for this webinar to obtain an overview of how Fc-mediated functions (or lack of) are essential to the success of biologic drugs and how best to evaluate these functions.
.
Speakers

Rosa Gonzalez-Serrano, Senior Manager, Abzena
Rosa Gonzalez-Serrano is a Senior Manager in the Bioassay group at Abzena, Cambridge. Her main role is in vitro characterisation of monoclonal antibodies and antibody conjugates, with a special focus on Fc effector function.
Rosa started her career working on small molecules at a global CRO and then moved to a large pharma company as a Pharmacodynamics (PD) Lead in Oncology. Rosa has a BSc from the University Rovira i Virgili, Spain, and a MSc (Hons) from the University of Groningen, The Netherlands.

Erika Kovacs, Senior Director, Abzena
Erika Kovacs leads the bioassay team at Abzena to support drug discovery and development efforts with cell-based assays for lead selection, characterization, potency and safety. Her expertise is in functional characterization of complex mode of actions and is passionate about finding new modalities to fight cancer.
Erika has a strong academic background obtaining a PhD from Eotvos Lorand University, Hungary, and doing a postdoc at University of California, Berkeley, prior to joining Abzena.
Who Should Attend?
This webinar will appeal to:
- Early-stage researchers from academia and small startups through to big pharma who are developing a biologic with an Fc domain
- Late-stage drug developers who require Fc-functional assessment and potency testing as part of CMC for their product
- Those working in Drug Development
- Those working in Biopharma/Biotech
- Those working in Cancer Research/Oncology
What You Will Learn
Attendees will learn about:
- Best approaches to assess Fc function (ADCC, ADCP and CDC) for drug discovery and development programs
- Benefits of working with experienced clinical research organizations (CROs) to ensure high-quality and reliable data are obtained
- Benefits and challenges of complex assay formats like real-time spheroid cytotoxicity assays
- How to design and develop successful Fc-mediated functional assays, including the selection of target cell lines, effector cells and appropriate controls
Xtalks Partner
Abzena
Abzena is as committed to success as you are. We have the capabilities, capacity and knowledge to move your asset forward as carefully and quickly as possible.
Our seasoned professionals offer the kind of personalized service and flexible working relationship that your demanding, time-sensitive programs deserve. We’re not afraid to question assumptions, and not shy about presenting new and better ideas.
And then, we deliver.
With research, development, and cGMP facilities across locations in San Diego, CA, Bristol, PA, and Cambridge, UK, Abzena is fully prepared to move your bioconjugate and biologics programs forward at every step in the process.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account





