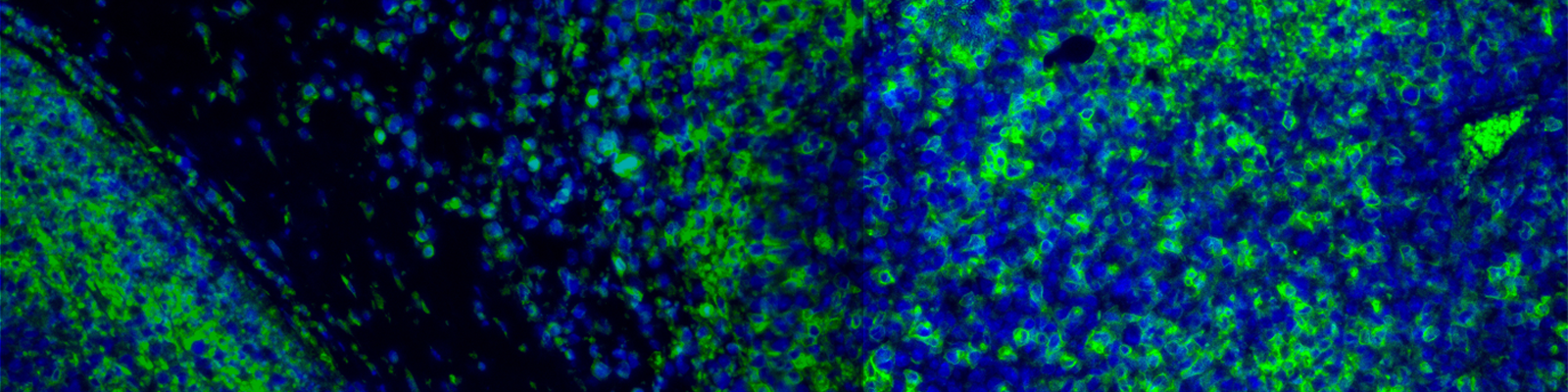
The extraction of quantitative pathology data endpoints from stained digitized slides is one of the most important steps when looking to answer specific questions spanning early discovery to late-stage clinical drug development. This applies to a wide-range of multi-omic and spatial-omic techniques, including multiplex chromogenic and fluorescent assays that aim to understand the contextual relationship between different cell phenotypes, such as immune cell colocalization with tumor cells.
The development, testing and validation of digital image analysis workflows is a complex process that requires a high-level of domain knowledge and technical expertise. There are many variables that can affect the results, including slide scanning platform parameters, approach to algorithm testing and training, analyst training, quality control methods, applying artificial intelligence (AI) and others. To address the variability and challenges, a fully customized approach can be developed based on the research question, clinical stage of development and intended use of the data.
Register for this webinar to hear experts review the current challenges encountered across the various stages of biomarker development, from early discovery to patient selection strategies in clinical trials. Specifically, they will discuss how applying state-of-the art computational digital pathology methods can uncover insights needed to accelerate the development of tailored therapies.
Speakers

Joseph Krueger, Ph.D., VP of Research & Applications, Invicro
Dr. Krueger serves as the Vice President of Research and Applications for Advanced Pathology Services at Invicro. In his role, Dr. Krueger is responsible for developing new biomarker applications to meet drug development needs, and provides scientific expertise needed to support sponsor-based projects across therapeutic areas. Prior to joining Invicro, Dr. Krueger was Chief Scientific Officer at Flagship Biosciences, Inc., where he led the R&D and Scientific efforts in applying novel quantitative tissue image analysis approaches to immuno-oncology and rare diseases to support drug and companion diagnostic development. Prior to Flagship Biosciences, he served in senior scientific roles in oncology drug development for OSI Pharmaceuticals in Boulder, CO and Pfizer in Andover, MA. Dr. Krueger completed his postdoctoral fellowship at The Scripps Research Institute and The University of California, San Diego after completing his Ph.D. in Cancer Biology at Wayne State University in Detroit, MI.

Omid Ghasemi, Ph.D., Senior Scientist, Invicro
Dr. Ghasemi is a Senior Imaging Scientist at the department of Advanced Pathology Services at Invicro. He has more than 7 years of research experience in imaging informatics and data analysis developing digital pathology pipelines for drug and biomarker development. Currently, Dr. Ghasemi is leading efforts to develop innovative image analysis applications and workflows across a wide breadth of histopathology projects for both clinical and preclinical studies. Prior to joining Invicro, he worked for three years as an Imaging Scientist on several drug research projects at different preclinical stages at Takeda Pharmaceuticals. Dr. Ghasemi completed his postdoctoral fellowship in mathematical modeling and imaging research at Merrimack Pharmaceuticals in Cambridge, MA. He holds a Ph.D. in Electrical Engineering with a concentration in image & signal processing and computational biology from The University of Texas at San Antonio.
Who Should Attend?
This webinar is recommended for pathologists and research scientists focused on drug discovery and development, which requires sophisticated data processing and analytics. By attending this webinar, attendees will better understand how domain and technical expertise is applied to design digital pathology analysis workflows to detect and quantify tissue biomarkers.
What You Will Learn
- Current challenges in image data processing and how domain expertise can be applied to address them
- Study design approach to reconcile multiple datapoints assessed and measured from each tissue section
- Algorithm development, testing and validation for specific image analysis applications
- Utility of techniques developed to generate application-specific proof points
Xtalks Partner
Invicro
Headquartered in Boston, MA, Invicro was founded in 2008 with the mission of improving the role and function of imaging in drug discovery and development across all therapeutic areas. Today, Invicro’s multi-disciplinary team provides solutions to pharmaceutical and biotech companies across all stages of the drug development pipeline (Phase 0-IV), imaging modalities and therapeutic areas, including neurology, oncology, and systemic and rare diseases. Invicro’s quantitative biomarker services, advanced analytics and AI tools, and clinical operational services are backed by Invicro’s industry-leading software informatics platforms, VivoQuant™ and iPACS®, as well as their pioneering IQ-Analytics Platform, which includes AmyloidIQ, TauIQ and DaTIQ.
As a CAP-accredited, CLIA-certified laboratory, Invicro offers a wide range of anatomical and molecular pathology services, including core histology, multiplex immunohistochemistry and immunofluorescence, RNAScope® ISH, BaseScope™ ISH, pathologist interpretation, data management, image analysis and Quanticell™, a proprietary nanoparticle-based immunohistochemistry detection technology. Our pathology service laboratories support projects spanning biomarker discovery to clinical trials and companion diagnostics. As part of the Konica Minolta precision medicine organization and with their sister company Ambry Genetics, Invicro develops and leverages the latest approaches in quantitative biomarkers, including imaging, quantitative pathology, and genomics. For more information, visit www.invicro.com.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account