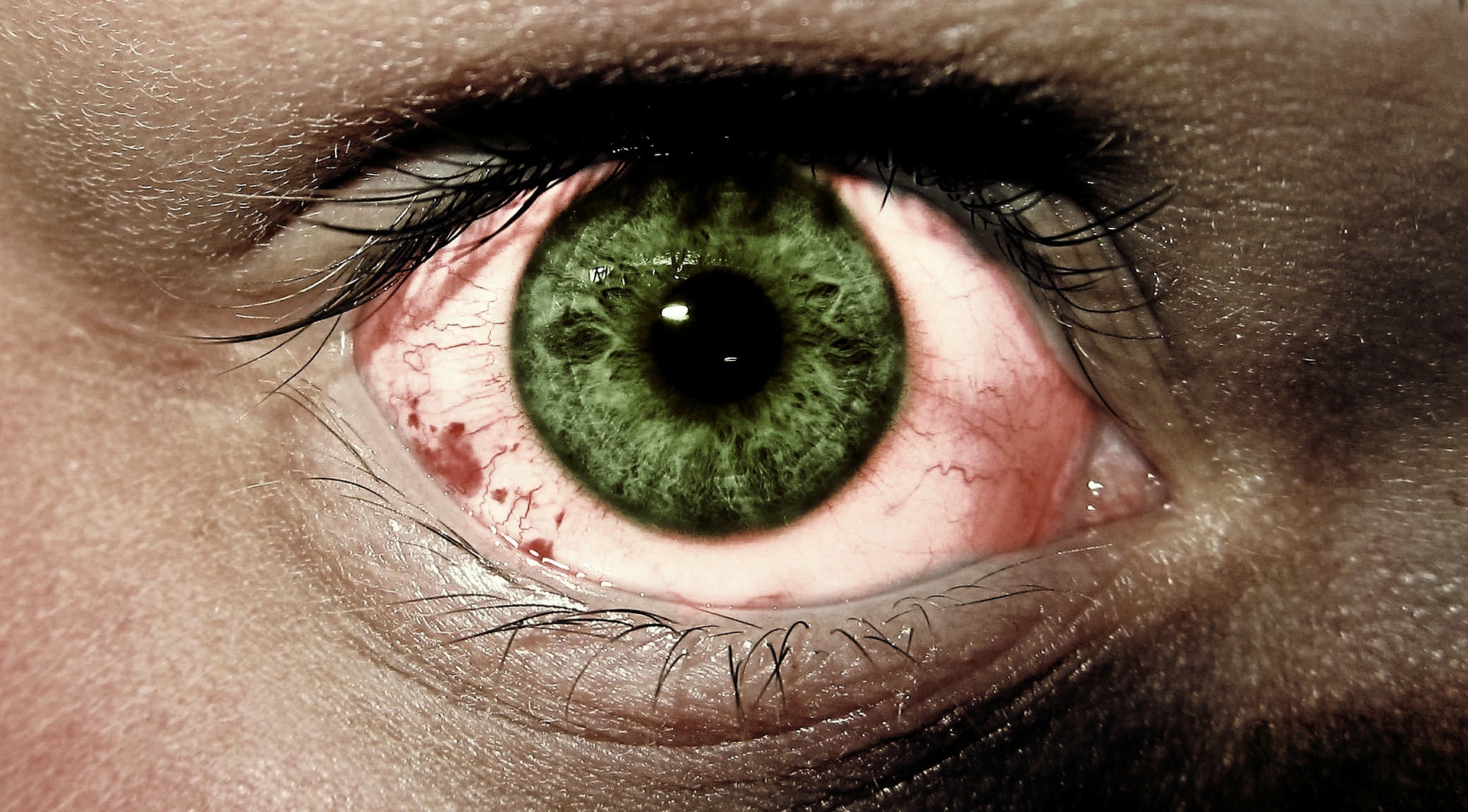
In time for World Glaucoma week, the US Food and Drug Administration (FDA) announced the approval of a new drug delivery system, Durysta for intracameral administration. This is the first biodegradable sustained-release implant for the treatment of open-angle glaucoma.
The 10 mcg implant works by administering the drug directly onto the anterior chamber of the eye where it releases bimatoprost. This allows it to continuously deliver the drug before dissolving entirely.
“Today’s FDA approval marks a breakthrough milestone for the glaucoma community and provides a much-needed option for patients challenged with topical drops or needing alternative options,” said David Nicholson, chief research and development officer at Allergan.
The new drug application was approved by the FDA based on results from two Phase III ARTEMIS studies. Durysta reduced the intraocular pressure by 30 percent over the 12-week efficacy period. After three doses, more than 80 percent of participants reported that they did not need any additional treatments, such as medicated eye drops, in order to help maintain their intraocular pressure for at least one year.
“Millions of people are living with glaucoma, one of the leading causes of vision loss; however, new treatment options are needed to help doctors and patients better manage this disease,” said Dr. Felipe Medeiros, Distinguished Professor of Ophthalmology and Vice-Chair for Technology, Director Clinical Research Unit, Department of Ophthalmology, Duke University.
More than three million Americans are living with glaucoma. Over two million of them are older than 40 years old and most commonly affected by open-angle glaucoma. According to Bright Focus, glaucoma is one of the leading causes of irreversible blindness. Moreover, there is no cure for glaucoma and vision cannot be regained.
The maker of Durysta, Allergen, is a pharmaceutical company that focuses on eye care. “At Allergan, our mission is to contribute meaningful strategies that help preserve people’s vision, while ensuring that therapies are mindful of the realities of administration and compliance…Allergan has five ongoing Phase 3 studies with Durysta to support further potential FDA label enhancement and rest of the world approvals,” said Nicholson.








Join or login to leave a comment
JOIN LOGIN