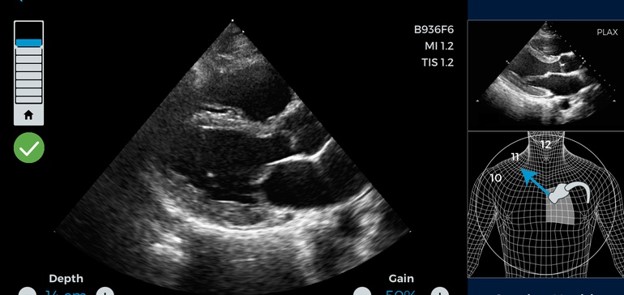
The US Food and Drug Administration (FDA) recently approved the first ever artificial intelligence (AI)-guided medical imaging tool for use in cardiac ultrasounds (i.e. echocardiogram or ECG). The tool, called Caption AI, was developed by the San Francisco-based AI company Caption Health.
Cardiac imaging is important in the assessment of heart problems, including cardiovascular disease. The new AI-guided tool may help revolutionize cardiac imaging by allowing health practitioners, including those that have no ultrasound experience, to obtain faster and more accurate images of the heart through automated technology. This will help increase the speed at which cardiac ultrasounds are performed, in addition to increasing the quality and accuracy of images due to the tool’s use of advanced AI technology.
Caption AI achieves a high degree of precision in its ultrasound images through AI-based guidance, precision automation, quality assessment and robust interpretation software that are built into the system. Moreover, because Caption AI is a tool that can be used by almost any type of health practitioner, ultrasound images can be attained more rapidly in clinical settings.
In a statement, Robert Ochs, Ph.D., deputy director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health Echocardiograms noted that, “Today’s marketing authorization enables medical professionals who may not be experts in ultrasonography, such as a registered nurse in a family care clinic or others, to use this tool. This is especially important because it demonstrates the potential for artificial intelligence and machine learning technologies to increase access to safe and effective cardiac diagnostics that can be life-saving for patients.”
Related: FDA Breakthrough Device: Artificial Intelligence Meets Cancer Pathology
Caption AI utilizes Caption Guidance Software, which uses artificial intelligence to give real-time feedback and guidance on images as they are acquired in order to optimize and enhance image quality. The FDA has just recently provided marketing authorization of the software.
A multi-center prospective trial at Northwestern Medicine and Minneapolis Heart Institute at Allina Health was conducted to evaluate the efficacy and safety of Caption Guidance among registered nurses who had no previous experience in ultrasound imaging. A panel of five expert cardiologists found that the exams were of good image quality, demonstrating the tool’s effectiveness and ease of use. The data from the study has been presented at international conferences including the American Heart Association (AHA) and the American Society of Echocardiography (ASE) and will soon be published in a peer-reviewed journal.
Caption AI also comes equipped with fully automated interpretation software called Caption Interpretation, which is now also FDA approved. The software is used to calculate a patient’s ejection fraction (EF), which is a common measurement to evaluate cardiac function. The software automatically collects and reviews clips from an exam and chooses the best ones to calculate EF. Caption Interpretation has been optimized using a training set consisting of approximately 9,000 patients and over four million images in total.
Through the use of sophisticated AI technology, Caption AI will help revolutionize cardiac imaging and help in the more rapid and timely diagnosis of heart conditions.






Join or login to leave a comment
JOIN LOGIN