Companies outsource to gain access to specialized expertise and technology, scale pharmacovigilance (PV) activities, and reduce costs. However, the efficiency and effectiveness of externalized safety activities are below expectations for many biotech and pharma companies. Modern cloud solutions are revolutionizing outsourcing by enabling seamless PV processes across internal and external parties, reducing or eliminating manual activities, and providing a single source of safety data and content for all parties.
Many legacy pharmacovigilance systems are fragmented with multiple applications, redundant safety data, and inefficient processes. Point solutions often do not work well together and are difficult to manage with multiple vendors and integrations. Data reconciliation and other non-value-added work become core activities to ensure information is accurate for reporting and decision-making. Streamlining processes and making collaboration easier with modern solutions enable companies to focus on higher impact activities.
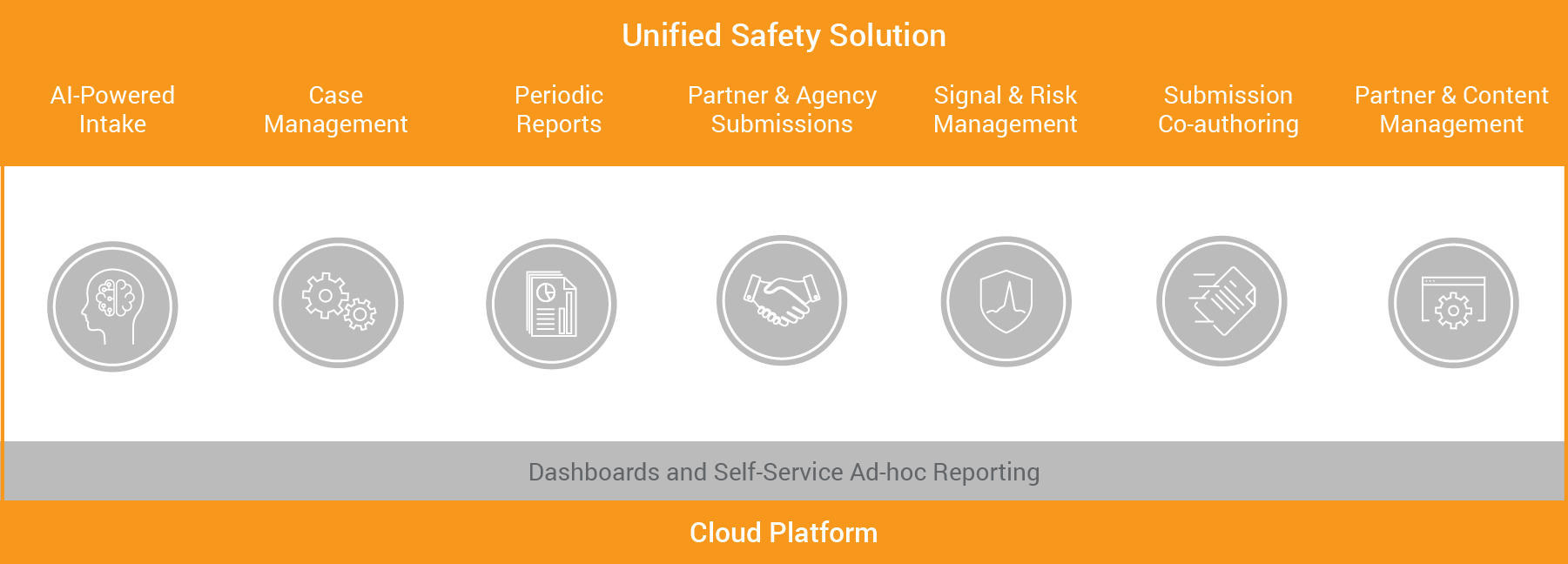
Transforming pharmacovigilance with a unified approach, modern safety applications leverage technologies such as cloud and artificial intelligence. With case intake, case processing, operational reporting and analytics, aggregate reporting, coding, signal and risk management, content management, and submissions in one solution, it is much easier to track and complete activities, process adverse events from intake to submissions, and analyze data for potential signals. Granular security and flexible processes also enable CROs, service providers, and pharma companies to all seamlessly work together in a single safety system.
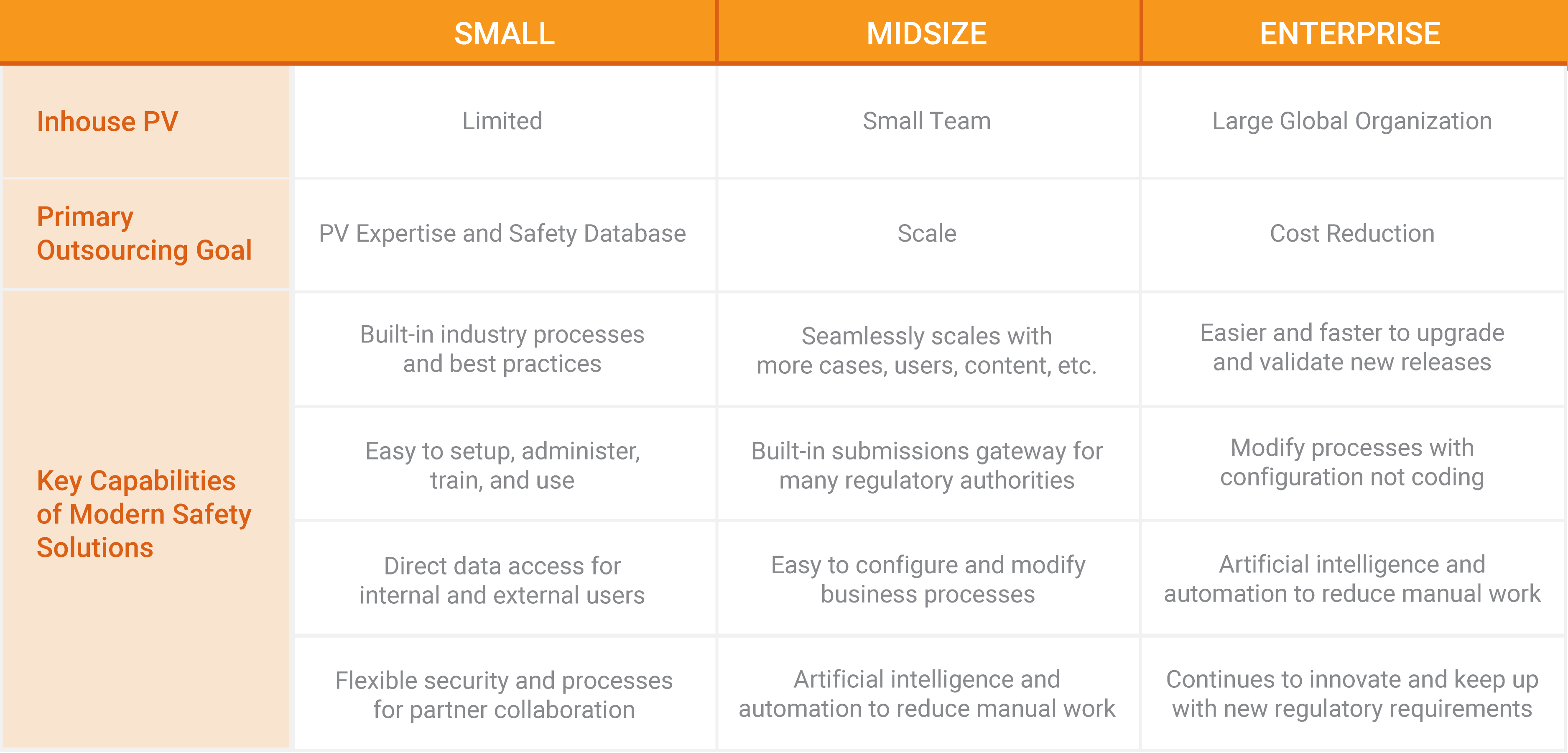
Small biotech and pharma companies have limited inhouse resources with safety knowledge and capabilities. With modern PV applications, companies can easily outsource safety services without losing control or visibility to the data. Cloud solutions provide greater transparency and collaboration between pharma companies and service providers or CROs for stronger alignment and partnerships. Midsize and large companies often have an internal drug safety team and safety database, and work with vendors to scale and reduce pharmacovigilance costs. With cloud safety applications, companies can seamlessly scale with more users, data, content, and cases as well as easily upgrade and validate new releases. Built-in submissions gateway for many regulatory agencies supports companies as they expand globally, and recent advancements in artificial intelligence and automation are enabling PV processes to scale with less overhead.
Leveraging modern safety applications in outsourcing strategies can increase the quality of execution by partners as well as mitigate risks and challenges the industry has historically faced when externalizing pharmacovigilance processes. Service providers and CROs adopting cloud technologies are able to:
- Make Compliance Easier – Modern solutions are easier and faster to set up and modify and have an intuitive user experience. Users require less training, are more productive, and can complete tasks in a timelier manner.
- Implement Scalable PV Processes – Designed to seamlessly and globally scale, cloud applications support companies as they grow with more cases, increasingly complex processes, or new markets.
- Reduce Costs – With greater automation and easier upgrades to new software releases to meet new and changing regulations, PV teams can reduce cost and risk and have more time to focus on high-value services.
Join Clinlogix to hear how they are driving greater transparency and efficiency with pharma and biotech companies, and improving pharmacovigilance outsourcing with modern technologies. Register for the webinar here.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN