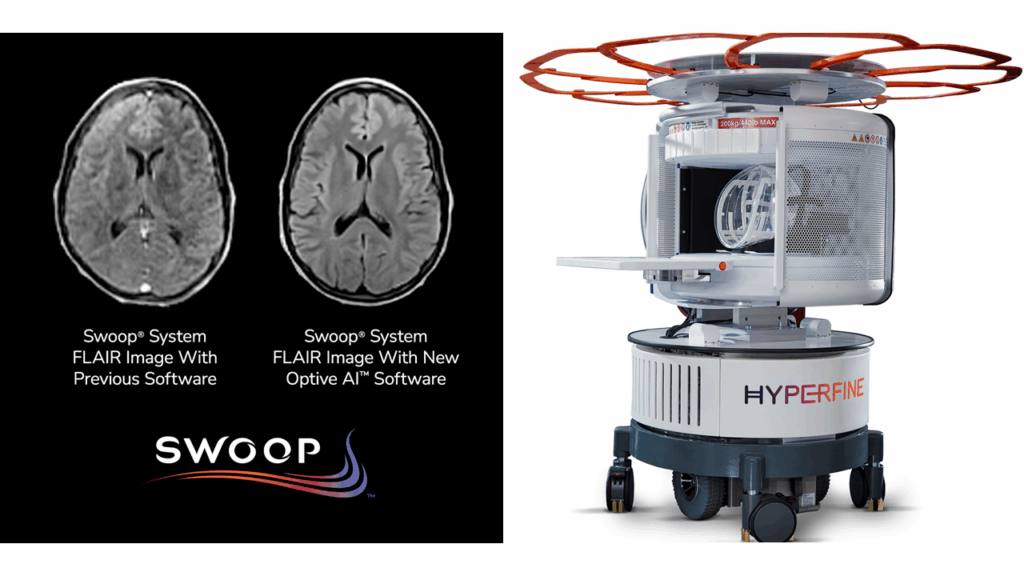
The FDA has cleared Optive AI software from Hyperfine, Inc., a health technology company pioneering portable brain imaging. This clearance opens new doors for clinical care, offering improved image quality for the company’s Swoop Portable MR Imaging system, which is FDA-cleared for brain imaging in patients of all ages.
At its core, the Swoop system combines a portable, hospital-based ultra-low-field MRI scanner with advanced AI-powered processing. Unlike conventional, stationary MRI machines, the Swoop system can be moved directly to the bedside within a hospital or clinical setting. This flexibility is crucial for patients who may be too unstable to travel to a traditional imaging suite, such as those in intensive care units or emergency rooms.
Research has shown that point-of-care (POC) bedside MRI in the ICU can shorten turnaround times compared to traditional MRI and reveal unique diagnostic findings, with 13% of bedside MRI scans detecting acute infarctions that CT scans missed — offering potentially real value in critical care.
A study in eClinicalMedicine demonstrated that AI-powered MRI analysis can uncover cardiac risk factors in stroke patients, highlighting the expanding clinical potential of these technologies.
Optive AI’s main strength lies in its ability to process and refine the images captured by Swoop’s ultra-low-field scanner. Using advanced algorithms, the software minimizes noise — unwanted visual artifacts that can obscure clinical details — while sharpening anatomical structures in the images. The improvements span all imaging sequences, enhancing clarity and uniformity from start to finish.
Related: How GE HealthCare’s SIGNA MAGNUS 3.0T Transforms Neuroimaging
Early clinical feedback has been encouraging. Some clinicians have said the new images generated by Optive AI approach the quality typically seen with standard 1.5 tesla MRI scanners. These enhancements not only improve diagnostic confidence at the point of care but also have the potential to transform patient management in settings where traditional MRI is not practical.
Tom Teisseyre, Hyperfine’s Chief Operating Officer, noted that these significant improvements in image quality are expected to accelerate Swoop’s adoption in hospitals and support its expansion into the neurology office market.
The company plans to begin deploying Optive AI to clinical accounts in the third quarter of 2025.
Across the broader portable MRI market, companies like France-based Chipiron are developing alternative methods to bring MRI to more patients, using a low-Tc SQUID volume gradiometer to capture ultra-sensitive magnetic signals. Their system, still in pre-clinical stages, aims to improve the signal-to-noise ratio up to tenfold compared to traditional low-field MRI.
Meanwhile, New Zealand-based medical device company Wellumio’s Axana device, which employs Pulsed Gradient Free Mapping (PGFM), is in clinical trials to evaluate its potential for bedside stroke detection.
Through this surge in innovation, the global POC imaging market is projected to grow at a compound annual growth rate (CAGR) of 4.3% from 2023 to 2030.
In April, Hyperfine launched the NEURO PMR study to directly evaluate Swoop’s image quality and clinical utility in private neurology practices, leveraging its AI-powered image reconstruction to compare performance with standard high-field MRI. More recently, the company announced a collaboration with NVIDIA to further accelerate image reconstruction using NVIDIA’s AI training tools, such as DALI and MONAI, and integrate real-time clinical decision support into the portable MRI workflow.
If you want your company to be featured on Xtalks.com, please email [email protected].










Join or login to leave a comment
JOIN LOGIN