Prostate cancer stands as the second leading cause of cancer death among American men. Its early detection enhances the likelihood of survival, and survival is greatly influenced by the type and localization of the cancer. As a result, there is a constant demand for highly specific molecular imaging technologies.
Recently, Blue Earth Diagnostics announced that the US Food and Drug Administration (FDA) has granted approval to its novel, high-affinity radiohybrid prostate-specific membrane antigen (PSMA)-targeted PET imaging agent, Posluma (flotufolastat F 18).
Posluma is now the first and only radiohybrid PSMA-targeted PET imaging agent to receive FDA approval.
Posluma is indicated for PET of PSMA-positive lesions in men with prostate cancer who:
- are suspected of having metastatic disease and are potential candidates for first-time definitive treatment
- are suspected of having a recurrence, as suggested by increased levels of serum prostate-specific antigen (PSA)
“With the FDA approval of Posluma, we realize our goal of providing an important product that will be widely available across the US to help inform the management and treatment of patients across the prostate cancer care continuum,” said David E. Gauden, CEO of Blue Earth Diagnostics, in the company’s news release.
XTALKS WEBINAR: Clinical Trial Assay Development for Precision Medicine: Insights from Pharma & Diagnostics Experts
Live and On-Demand: Wednesday, June 21, 2023, at 11am EDT (4pm BST/UK)
Register for this free webinar to learn about the opportunities and challenges of clinical trial assay development & validation and the critical steps involved to ensure that they are ready for stratification use within clinical trials.
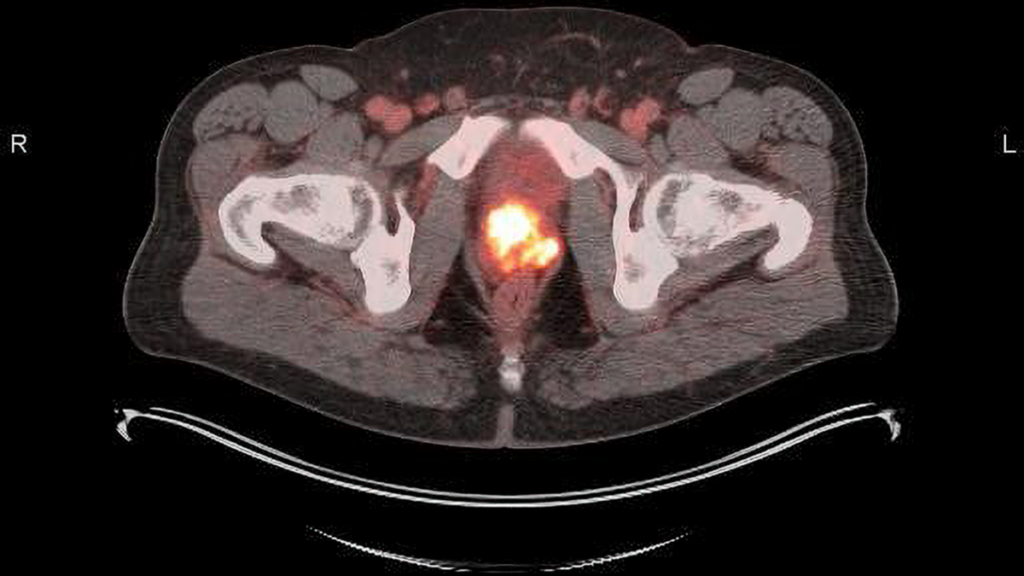
How Does Posluma Work?
Posluma contains a radioactive imaging agent, fluorine-18 (18F), that is designed to specifically bind to the PSMA over-expressed on prostate cancer cells. When injected into a patient’s bloodstream, Posluma selectively accumulates in PSMA-expressing prostate cancer cells. The attached radioactive tracer emits positrons, which can be detected by a PET scanner, enabling physicians to accurately identify and locate prostate cancer cells both within the prostate and throughout the pelvis and the body when tumors have migrated.
Is Posluma Safe and Effective?
The FDA’s approval of Posluma was based on two Phase III clinical trials, LIGHTHOUSE and SPOTLIGHT.
The LIGHTHOUSE study demonstrated high specificity for detecting prostate cancer in 296 patients with unfavorable, intermediate-risk or high-/very high–risk prostate cancer across results from three central, blinded readers. It also showed high specificity in detecting pelvic lymph nodes compared to surgical pathology at a patient level in men with PSMA-positive lesions prior to radical prostatectomy.
The SPOTLIGHT study focused on the safety and diagnostic performance of Posluma in suspected prostate cancer recurrence based on elevated PSA following prior therapy. It displayed high detection rates even at low PSA levels.
A total of 747 patients with either initial or recurrent prostate cancer participated in these clinical trials, which evaluated the safety of Posluma. Severe adverse reactions were minimal, with diarrhea reported in 0.7 percent of patients, increased blood pressure in 0.5 percent and pain at the injection site in 0.4 percent. Notably, the agent carries a warning about radiation risk and the possibility of false positive results. A clinical correlation, which may include histopathological evaluation, is recommended.
Alternatives to Posluma
Posluma isn’t the only PSMA-PET imaging agent available. Ga 68 PSMA-11 and Pylarify (piflufolastat F 18) were approved by the FDA in 2020 and 2021, respectively.
Furthermore, a theranostic agent named Pluvicto, a PSMA-targeted radionuclide from Novartis for men with metastatic castrate-resistant prostate cancer, was approved by the FDA in March 2022.
Present and Future Prospects for the Imaging Agents Market
In 2021, the market value of imaging agents was reported to be over $9.2 billion, and it is projected to grow at a compound annual growth rate (CAGR) of 5 percent, potentially surpassing $14.2 billion by 2030, according to a report by Global Market Insights. This market is primarily driven by advancements in technology, the rising prevalence of chronic diseases, the increasing demand for early diagnosis and the necessity for personalized medicine.
R&D efforts ongoing in this field are anticipated to further boost market growth in the coming years. Major industry players, including GE Healthcare, Eli Lily, Bayer Healthcare Pharmaceuticals, Alliance Medical, Bracco Diagnostics and others, are significantly investing in R&D, as well as engaging in strategic collaborations and acquisitions to stay competitive and enhance their market value.
However, the market’s success hinges on how these companies navigate challenges such as the high cost of imaging agents and procedures, as well as safety concerns linked with the use of these agents and diagnostic imaging procedures.











Join or login to leave a comment
JOIN LOGIN