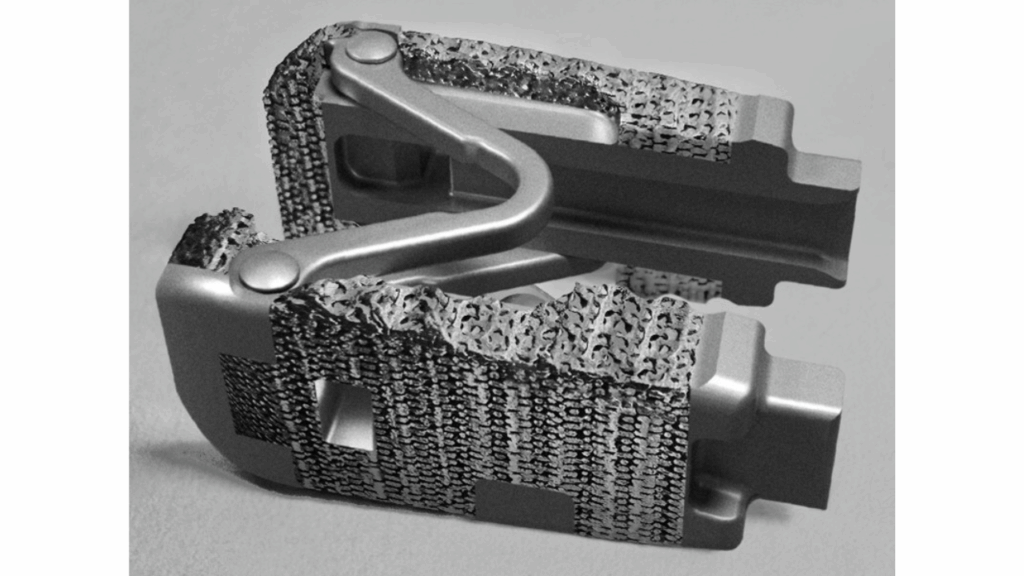
Spine Innovation has received FDA 510(k) clearance for its LOGIC titanium expandable interbody system. The device is a next-gen version of the company’s LOGIC spinal fusion implant. It is intended for use in interbody fusion procedures, in which a device is placed between two vertebrae to help stabilize the spine and promote bone growth.
The cleared system incorporates OsteoSync Ti, a porous, pure titanium lattice material developed by Sites Medical. According to the company, this material has been implanted in more than 250,000 patients since 2014 across orthopedic and spine applications.
The lattice is designed to support bone ongrowth and ingrowth, meaning bone can attach to and grow into the implant surface. It is also intended to provide the mechanical strength needed to support the spine during fusion.
The LOGIC implant uses an expandable design. It is inserted with a reduced profile and then expanded in situ to more than double its original size. The expansion is intended to restore disc height and help re-establish sagittal balance, or the spine’s natural front-to-back alignment. It also creates space for bone graft material.
When fully deployed, the implant has an expanded footprint of 18 mm by 26 mm across the vertebral endplates. A larger surface area is intended to distribute loads more evenly and reduce the risk of subsidence, or sinking into the bone, as well as implant migration.
The expandable LOGIC design has been used clinically in the US for about a decade in a version made from polyether ether ketone (PEEK). With the current clearance, the same design is now available in a porous titanium format. This provides surgeons with an alternative material option while retaining the established mechanical and geometric features of the earlier system.
Spine Innovation was founded in 2013 in collaboration with surgeon-inventors from Scripps Health in San Diego.
Related: Medical Device and Medtech IPOs of 2025: Driving Smarter, More Personalized Care
Other New Developments in Interbody and Vertebral Body Implants
Other spine device companies are also advancing new interbody and vertebral body implant technologies, with a focus on expandable designs, porous or bioactive materials and improved integration with bone. In late 2025, HAPPE Spine reported that its INTEGRATE-C interbody fusion system, which uses a proprietary bone-integrating biomaterial, had been implanted in more than 1,000 patients across multiple US centers.
Some groups are also exploring how lattice structures and advanced materials could support both mechanical stability and biological response. In mid-2025, NanoHive Medical reported research efforts to integrate piezoelectric sensors — devices that generate electrical signals in response to mechanical stress — into its 3D-printed Soft Titanium interbody implants, with the goal of enabling bone stimulation and remote monitoring. The program was described as being in early development and regulatory engagement.
On the regulatory side, Vy Spine’s VyBrate vertebral body replacement system was FDA-cleared for use when an entire vertebral body must be removed and replaced. The system combines a porous lattice architecture with a PEKK-based material, a high-performance polymer related to PEEK, designed to support bone attachment and imaging visibility in cervical and thoracolumbar reconstruction.
Around the same time, Spineology launched its OptiMesh HA Nano expandable interbody system, incorporating a nano-scale hydroxyapatite surface coating, a bone-like mineral layer intended to encourage bone growth onto the implant surface.
For Spine Innovation, the FDA clearance means the LOGIC expandable spinal fusion implant is now available in a porous titanium version.
If you want your company to be featured on Xtalks.com, please email [email protected].












Join or login to leave a comment
JOIN LOGIN