A medical device startup has begun enrolling patients into a clinical trial for a light-based therapy designed to treat a common form of age-related macular degeneration (AMD).
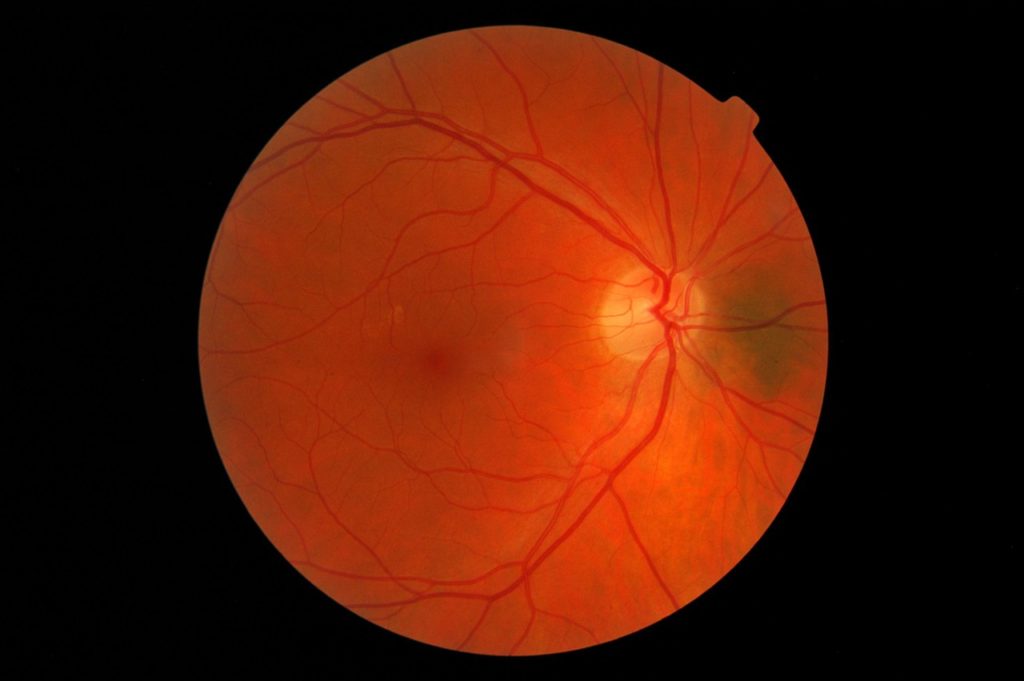
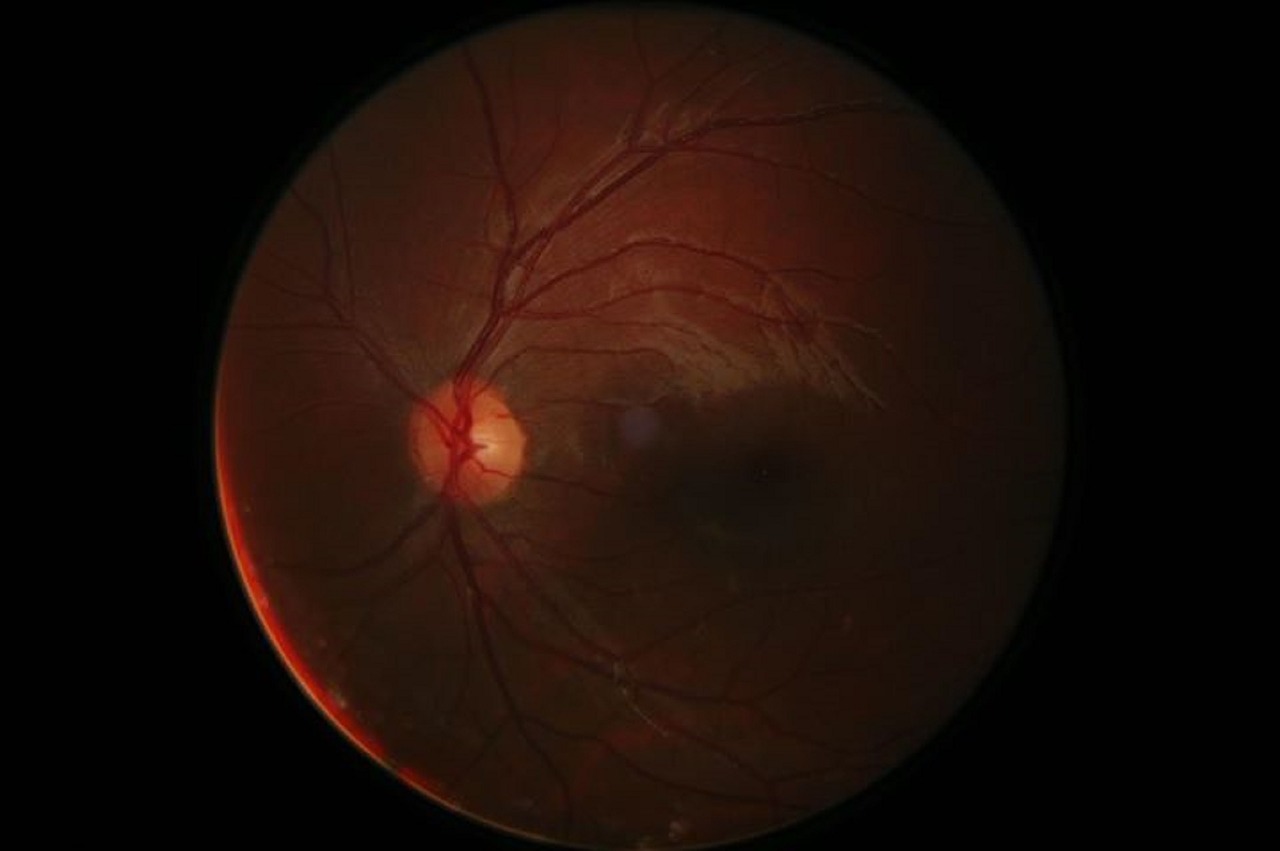
AMD is a leading cause of vision loss and blindness in Americans over the age of 65, according to the Centers for Disease Control and Prevention. A majority of patients have the “dry” form in which deposits known as drusen develop and cause the delicate retina to deteriorate. Despite the prevalence of this disease, there are few effective treatment options.
However, nonpharmaceutical interventions such as dietary supplements, lenses and lasers could help slow or reduce the risk of AMD. Washington-based LumiThera is leveraging a technique known as photobiomodulation, which uses a low-level light to stimulate the growth and proliferation of retinal cells to treat dry AMD.
“This is an exciting opportunity to establish a potential new treatment for dry AMD patients,” said Dr. Allen Hu, in a statement. He added, “This study critically provides an important medical device treatment for the majority of patients with intermediate dry AMD and limited options.”
Dr. Hu is the principal investigator of Lightsite III, the randomized, multi-center prospective clinical study evaluating LumiThera’s photobiomodulation device, the Valeda Light Delivery System. The first of 100 patients with dry AMD were enrolled into the study last week.
Patients will be treated with photobiomodulation or a sham device over the course of two years. The team will be evaluating visual acuity, contrast sensitivity and reduction of drusen deposits among other outcome measures.
LumiThera could be the first to commercialize a photobiomodulation-based treatment for dry AMD in the US, if approved by the US Food and Drug Administration (FDA). The device is already approved for commercial use in the European Economic Area and a post-marketing, multi-center trial was initiated earlier this year.
Photobiomodulation is also being investigated in non-ocular conditions such as chronic pain, arthritis and neurological disease. NovoTHOR’s whole-body photobiomodulation device was developed to relieve muscular and joint pain, and has been well-received by high-performance athletes and individuals suffering from chronic pain.
This form of light therapy is thought to activate energy production via the mitochondria, the so-called powerhouse of the cell. Its application in sports medicine and pain relief is obvious, but perhaps less understood in the treatment of retinal diseases. Retinal cells are one of the most energy-dependent cells of the body, thus targeting energy production and promoting cell survival could be key to slowing or possibly reversing vision loss.
Currently, drug giants Novartis and Regeneron Pharmaceuticals dominate the wet AMD market with Lucentis and Eylea, respectively. But with the AMD and retinal disease drugs market expected to grow to $21.42 billion by 2023 (as reported by VisionGain), LumiThera could potentially become a leader in the dry AMD treatment space.








Join or login to leave a comment
JOIN LOGIN