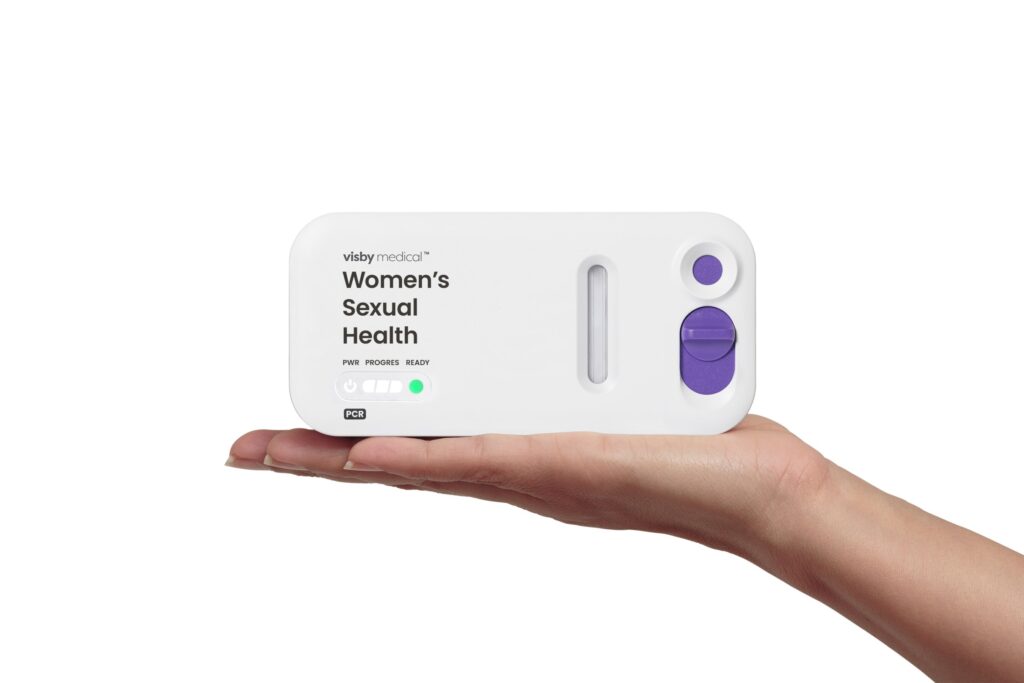
The FDA has granted De Novo authorization to the Visby Medical Women’s Sexual Health Test.
The PCR-based test detects three of the most common and curable STIs: chlamydia, gonorrhea and trichomoniasis.
The single-use test, designed for women and regardless of symptom presence, delivers results in approximately 30 minutes.
It includes a self-collected vaginal swab and a powered testing device. The test securely communicates with the companion Visby Medical App to display results upon completion.
“This approval is not just a milestone for Visby Medical but marks a transformative moment in medical diagnostics,” said Adam de la Zerda, PhD, founder and CEO of Visby Medical.
He said the palm-sized device replaces bulky, large, expensive laboratory instruments.
“After 12 years of development, our device delivers rapid, reliable results directly into the hands of consumers, with unparalleled convenience and privacy. We also built a state-of-the-art, fully automated manufacturing line ready to rapidly scale production in anticipation of growing consumer demand,” he explained.
The approval follows a previous FDA nod for a point-of-care version of the test used by healthcare professionals.
Related: Mira’s New Menopause Transitions Kit Allows for At-Home Hormone Monitoring
“Home tests can give people information about their health from the privacy of their home. This can be particularly important for sexual health tests for which patients may experience fear or anxiety, possibly resulting in delayed diagnosis or treatment,” said Courtney Lias, PhD, director of the FDA’s Office of In Vitro Diagnostic Devices.
According to the CDC, more than 2.2 million cases of chlamydia and gonorrhea were diagnosed and reported in the US in 2023.
Additionally, trichomoniasis is estimated to affect approximately 2.6 million people in the US. It is the most prevalent non-viral STI nationwide.
All three infections are typically treatable with antibiotics. If left untreated, they can lead to serious health complications, including infertility.
The Visby Medical Women’s Sexual Health Test offers a private and convenient option for at-home STI testing.
The user-friendly companion app guides users through the testing process, from sample collection to running the test and understanding the results. It also offers easy access to follow-up care if needed.
Visby said clinical studies with over 2,000 lay users evaluated the Women’s Sexual Health Test. The results showed accuracy on par with traditional lab-based PCR testing.
The FDA’s authorization was based on clinical studies that demonstrated high accuracy.
Among individuals with and without symptoms, the Visby Medical Women’s Sexual Health test correctly identified Chlamydia trachomatis in 98.8% of negative and 97.2% of positive samples.
The test correctly identified 99.1% of negative samples and 100% of positive samples for Neisseria gonorrhoeae.
And for Trichomonas vaginalis, the test correctly identified 98.5% of negative and 97.8% of positive samples.
Visby advises individuals who test positive to seek medical care.
The FDA reviewed the test under the De Novo premarket review pathway.
“The clinical significance of bringing a rapid, highly accurate PCR diagnostic test into the home environment cannot be overstated,” said Gary Schoolnik, MD, chief medical officer of Visby Medical.
“Extensive clinical studies validate that this test empowers women to quickly understand what steps to take next, giving them the privacy, control and confidence to seek the care they need. Importantly, many patients infected with these STIs are non-symptomatic, yet they can still suffer serious long-term health consequences. Our test directly addresses this silent epidemic by enabling detection and treatment.”
The clearance builds on other recent regulatory nods. This includes last year’s authorization of the first at-home syphilis test developed by NOWDiagnostics.
In 2023, the FDA granted marketing authorization to the first diagnostic test for chlamydia and gonorrhea using at-home sample collection.
The authorizations reflect the FDA’s ongoing expansion of approvals for self-collected samples in the diagnosis of STIs beyond HIV.
Last month, Visby also received FDA 510(k) clearance for its point-of-care respiratory test. The test detects influenza A and B as well as the SARS-CoV-2 virus that causes COVID-19. The PCR-based test had been granted emergency use authorization during the pandemic.
Visby said the latest FDA authorization of its triple STI test marks a major milestone for the company.
Visby said it is planning to expand the lineup of over-the-counter tests to include those for respiratory infections, sore throat conditions, urinary tract infections and other common illnesses.
“This is just the beginning of our journey to transform healthcare with at-home diagnostics,” said Dr. de la Zerda. “We’ll soon be announcing several strategic and commercial partnerships.”
In 2022, Visby raised $135 million in two funding rounds to support its transition beyond COVID-19 and broaden its portfolio of home-based and point-of-care diagnostic tests. The funding aimed to help the San Jose, California-based startup expand into new areas of point-of-care and home-based tests.










Join or login to leave a comment
JOIN LOGIN