Neuromuscular disorders (NMDs) are generally variably progressing, rare diseases causing devastating skeletal muscle injury resulting in severe disability and even death. In the intensive search for treatments and cures, MRI has emerged as a promising clinical and drug development tool that can detect early disease progression and treatment effects before loss of muscle function or disability can be observed with clinical assessments.
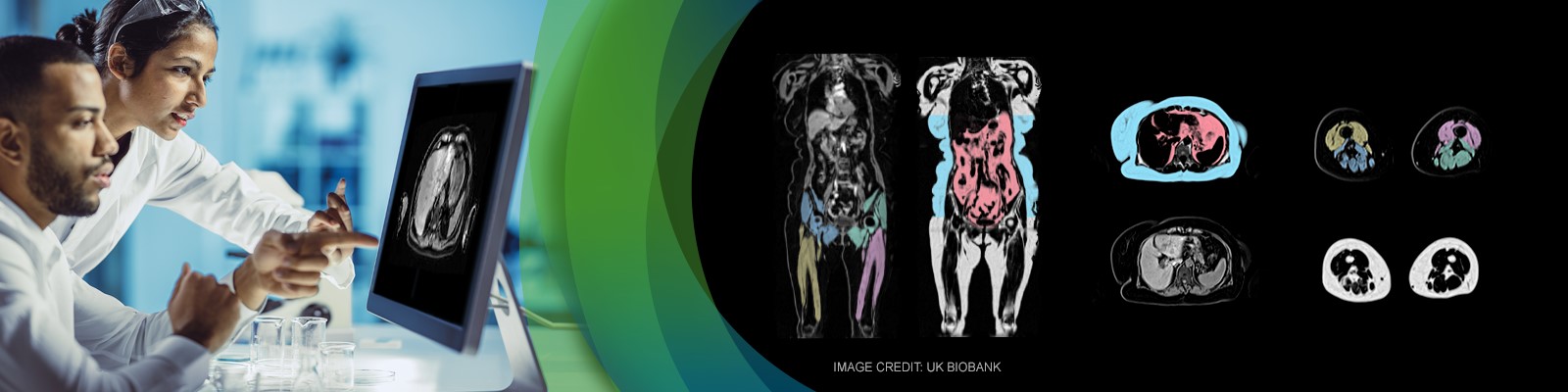
Muscle atrophy and muscle fat replacement are hallmarks of NMDs. Most NMDs are characterized by heterogeneity in the pattern and timing of muscle involvement among patients. This causes significant variability and leads to challenges in the assessment of potential treatment effects. Recent technical advances have led to the development of muscle biomarkers based on whole-body water and fat separated MRI that can describe the distribution and progression of muscle atrophy and fat replacement with high precision and accuracy. Imaging the whole body enables the identification of a personalized set of affected muscles, decreasing the variability and increasing the sensitivity of efficacy assessment.
In this webinar, two experts will review techniques for whole-body MRI and its application in NMD clinical research. Technical aspects will be discussed, including:
- Practical implementation of MRI scanning protocols
- Image analysis techniques
- Biomarker definitions
- How challenges related to multi-site implementation can be addressed
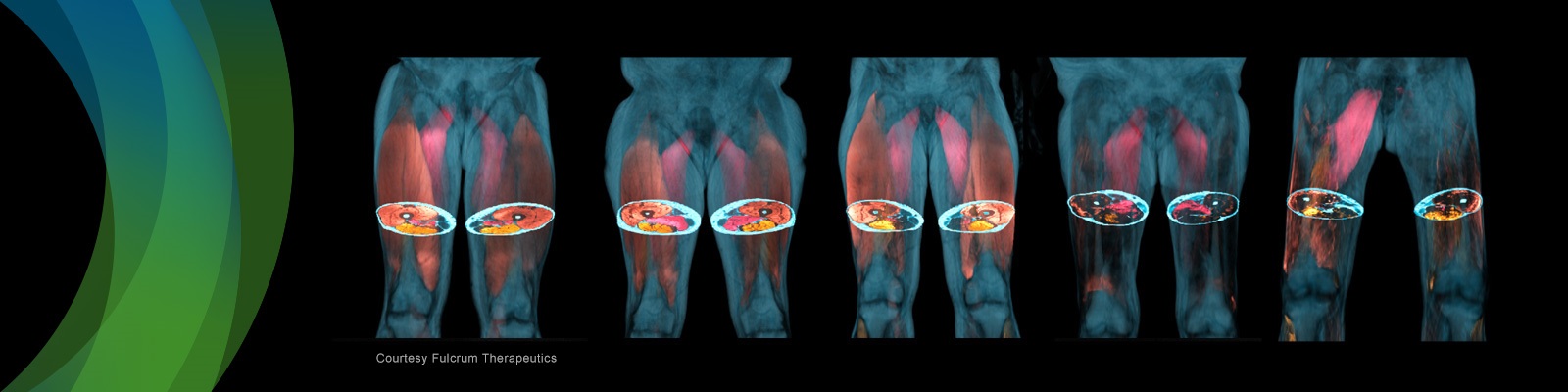
Further, featured speakers will discuss how measurements from multiple muscles based on a whole-body MRI scan can be combined into composite scores optimized to describe specific aspects of disease onset pattern, functional impairment, and disease progression between scans. Finally, experiences from recent clinical trials in facioscapulohumeral muscular dystrophy (FSHD) will be discussed exemplifying how such composite scores can be used as endpoints.
Register for this webinar to learn how whole-body MRI can support NMD clinical research.
Speakers

Markus Karlsson, PhD, Clinical Science Lead, AMRA Medical
Markus Karlsson, PhD, has over 6 years of research experience in the clinical applications of quantitative MRI. As a clinical scientist at ARMA Medical, he focuses his research on how AMRA’s technical platform can be applied as endpoints in clinical trials for neuromuscular disorders. Before joining AMRA, Markus completed his PhD at Linköping University, writing a thesis on quantitative MRI of the liver.

Michelle L. Mellion, MD, Executive Medical Director, Head of Neuromuscular Clinical Development, Fulcrum Therapeutics
Dr. Mellion is an executive medical director and head of neuromuscular clinical development working on therapies for rare neuromuscular diseases at Fulcrum Therapeutics. She graduated from Colgate University with honors in molecular biology and Wake Forest University Medical School. She went on to complete her internship in medicine, residency in neurology and fellowship in clinical neurophysiology at Brown. She is board certified in neurology and clinical neurophysiology. After approximately a decade on the faculty at the Warren Alpert Medical School/Brown University where she conducted her own grant-funded research, she served as director of the clinical neurophysiology fellowship, neurology residency program and was an attending physician at the MDA neuromuscular clinic as well as at her own private general neurology clinic.
Following this, Dr. Mellion decided to expand her scope of expertise in the pharmaceutical industry. She transitioned from academia to clinical development at Biogen where she worked on the remyelination program as well as contributed to the development of novel biomarkers. She then co-led the pain program at Vertex Pharmaceuticals. Dr. Mellion has authored and co-authored numerous publications on her research as well as in programs to which she has contributed in industry. She continues to see neuromuscular patients in her clinic affiliated with Tufts University.
Who Should Attend?
This webinar will appeal to individuals with the following or related job titles:
- Chief Medical Officer
- Chief Executive Officer
- Neuromuscular Clinicians
- Principal Investigator
- Medical Director
- Research Nurse
- Clinical Director
- Clinical Project Manager
- Imaging Specialist
- Clinical Operations
- Clinical Development Manager
- Physical Therapist
- Occupational Therapist
- Procurement Manager
- Statistician
- Data Manager
- Regulatory Coordinator
- Business Development
What You Will Learn
- Advantages of whole-body MRI techniques for NMD research
- Addressing multi-site MRI scanning protocol implementation
- Exploring early disease progression and treatment effects with MRI
- Discuss the heterogenous presentation of most NMDs
- Using composite scores as endpoints in recent FSHD clinical trials
Xtalks Partner
AMRA
AMRA Medical is a digital health company at the forefront of medical imaging and precision medicine. The company has developed a new global standard in body composition analysis, delivering multiple fat and muscle biomarkers with unrivaled accuracy and precision – all from a rapid whole-body MRI scan.
AMRA offers medical device and medical research services to support transformative care and vital decision-making, from clinical research to clinical care.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account