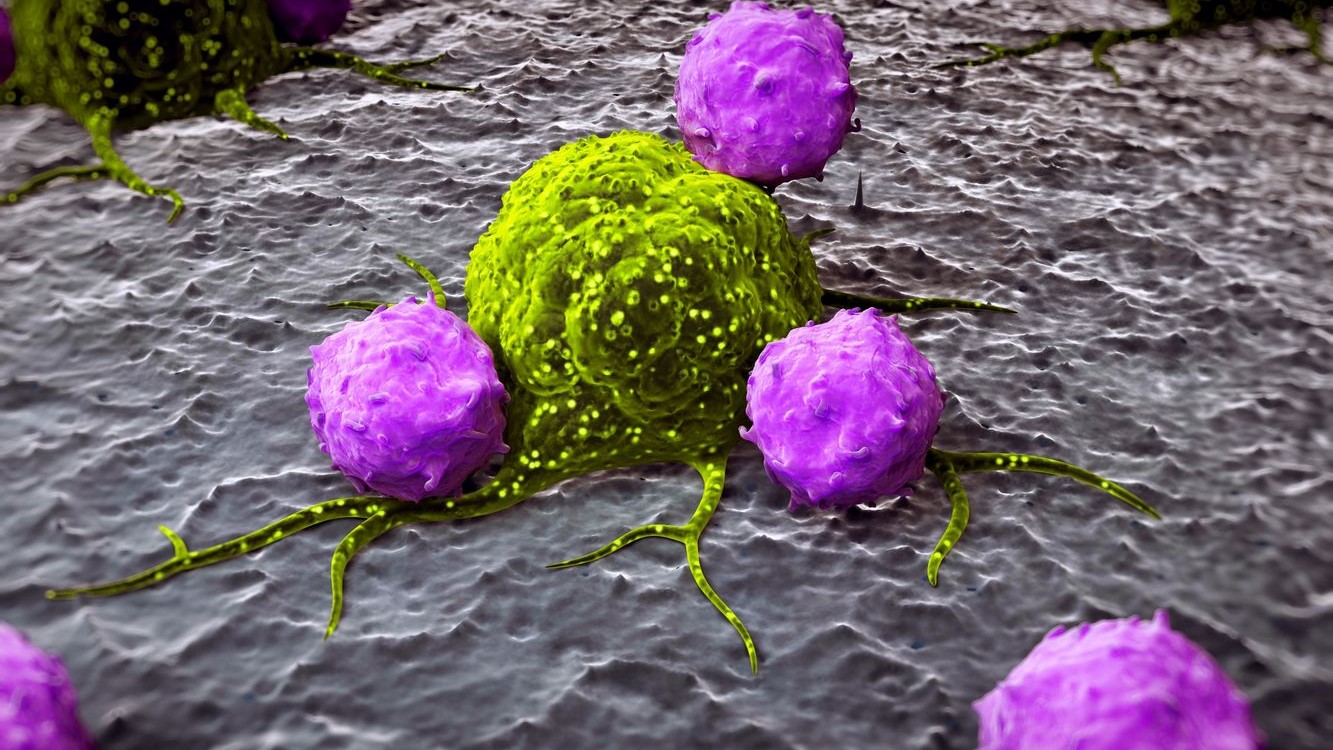
Macrophages are white blood cells that specialize in the detection, phagocytosis and destruction of bacteria and other harmful organisms. They can also present antigens to T cells and initiate inflammation by releasing cytokines, which activate other cells.
In other words, when fighting bacteria, the macrophages are programmed to be pro-inflammatory. However, when they are programmed to be anti-inflammatory, they can help decrease inflammation.
The anti-inflammatory macrophages help in preventing an immune response from becoming dangerous, as seen with patients with autoimmune disease. This helps to ensure that inflammation naturally subsides after the initial immune response and promotes tissue repair.
The study was done on an animal lung injury model that focuses on macrophage programming. They found that the process is more complex than what was previously studied. This is because macrophages are driven by more than just the immune system alone but by the microenvironment.
“We found that macrophage programming is driven by more than the immune system — it is also driven by the environment in which the macrophages reside,” said lead study author Asrar Malik, the UIC Schweppe Family Distinguished Professor and head of pharmacology and regenerative medicine at the College of Medicine.
In the study they “suggest that immune-mediated mechanisms of regeneration and repair may complement existing stem cell therapies or may be a viable alternative to using stem cells as a way to promote functional regrowth of vital tissues.”
Furthermore, the study “demonstrated that lung endothelial cells – which are the cells that line blood vessels – are essential in programming macrophages with potent tissue-reparative and anti-inflammatory functions,” said Dr. Jalees Rehman, UIC professor of medicine and pharmacology and regenerative medicine and co-lead author of the paper.
Researchers studied the proteins that act as chemical signals and conducted experiments to see how those signals were affecting the macrophages. They found a protein called Rspondin3, which is released at high levels during inflammatory injury. This protein plays a crucial role in macrophage programming.
“When we removed the gene responsible for Rspondin3 from the blood vessel endothelial cells, we observed that macrophages did not decelerate inflammation. Instead, the lungs became more injured,” said Bisheng Zhou, UIC research assistant professor of pharmacology and regenerative medicine and first author of the study. “We tried this in multiple models of inflammatory lung injury and found consistent results, suggesting that blood vessels play an important instructive role in guiding the programming of macrophages.”
Researchers found that the majority of people recover from a lung infection, but others could develop severe lung injury known as Acute Respiratory Distress Syndrome, ARDS. Moreover, due to the recent COVID-19 pandemic, researchers seen that that patients who have underlying undiagnosed poor vascular health, result in blood vessels failing to send the appropriate cues to the macrophages and turn off the inflammation.
“The lack of an adequate automatic braking system to slow inflammation once the bacteria or viruses have been eliminated leads to a situation in which our body’s own unchecked immune system becomes the cause of even greater damage to vulnerable tissues and organs such as the lung,” said Rehman.
The study may have focused on lungs, but the findings are also relevant to other organs such as the heart, intestines, brain and liver. These are all organs where immune cells can cause damage if the necessary balance between pro-inflammatory and anti-inflammatory cells is disrupted.
The study states, “evolution clearly selected fast healing and containment of injury or infection at the expense of the ability to reform a completely functional tissue. In order to selectively undo the loss of regenerative capacity without compromising the specificity and strength of the mammalian immune system, much remains to be learned about the functions of immunity, good and bad, in development, homeostasis and injury.”








Join or login to leave a comment
JOIN LOGIN