Rare and rheumatic disease-focused biotech Horizon Therapeutics has received expanded approval from the US Food and Drug Administration (FDA) for its gout injection Krystexxa (pegloticase) to include its co-administration with the immunomodulator methotrexate.
In a news release, Horizon said the drug combo can help more people with uncontrolled gout achieve a complete treatment response. Uncontrolled gout is defined as gout that is unmanageable and/or develops resistance to conventional therapies.
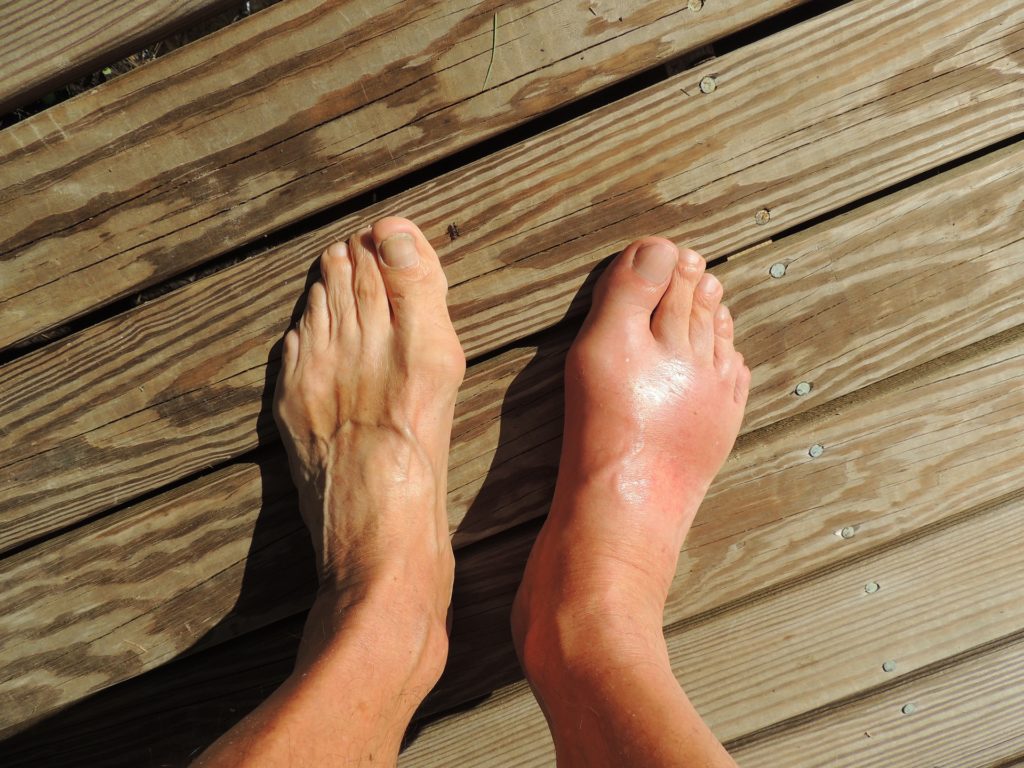
Gout is a type of inflammatory arthritis caused by the buildup of uric acid crystals in the joint, causing pain and inflammation. Krystexxa works to decrease serum levels of uric acid.
Krystexxa’s expanded approval was based on data from the randomized, controlled MIRROR trial involving adults with uncontrolled gout. Results from the trial showed “significant improvement and sustained response” to Krystexxa with methotrexate (a chemotherapy drug and immune suppressant) compared to Krystexxa alone.
Over time, even the use of Krystexxa can lead to the development of antibodies against it, reducing its effectiveness. This is why methotrexate was paired with it, as methotrexate is used to treat inflammatory conditions like arthritis. Early data also showed that methotrexate could prevent the formation of anti-drug antibodies.
In March, Horizon received FDA priority review for its supplemental Biologics License Application (sBLA) for the expanded labelling of Krystexxa.
XTALKS WEBINAR: Beyond the EHR: Clinical Trials in the Age of Abundant Data
Live and On-Demand: Tuesday, August 23, 2022, at 12pm EDT (5pm BST/UK)
Register for this free webinar for expert insights and an engaging discussion surrounding the utilization of electronic health record (EHR) data in clinical research and what the future might hold. The featured speakers will discuss what the right data partnerships can accomplish, from patient-centric protocols to artificial intelligence (AI)-run medical record retrieval.
Krystexxa is administered as an intravenous infusion every two weeks. It is the first and only biologic approved for the condition.
Krystexxa is a recombinant porcine-like uricase that metabolizes uric acid to allantoin. The drug is used as a third line treatment for severe chronic gout in individuals who have failed, or are unable to tolerate, other treatments (namely xanthine oxidase inhibitors) for uncontrolled gout. The condition affects about two to six percent of gout sufferers.
“Uncontrolled gout carries serious, long-term consequences in the joints and throughout the body, as well as a significant impact on a person’s daily life,” said co-primary investigator John K. Botson, MD, RPh, CCD, president, Alaska Rheumatology Alliance and rheumatologist, Orthopedic Physicians Alaska.
“Through multiple in-practice case series, the open-label trial and the randomized controlled trial, the medical community has been actively engaged in finding ways to reduce the impact of uncontrolled gout by maximizing the use of Krystexxa. The expanded labeling reflects robust data on this treatment approach, which can allow us to change outcomes for many uncontrolled gout patients, most of whom have no other treatment option.”
The MIRROR trial evaluating Krystexxa with methotrexate involved 100 adults living with uncontrolled gout that were randomly administered methotrexate (15 mg/week) or placebo for four weeks, and then treated with Krystexxa in combination with methotrexate or Krystexxa with placebo for 52 weeks. The primary endpoint was the proportion of serum uric acid (sUA) responders (sUA less than 6 mg/dL at least 80 percent of the time) at month six.
Results showed that there was a more than 30 percentage point increase in response rate during month six and this improvement was sustained through to month 12. Specifically, 71 percent of patients in the Krystexxa-methotrexate combination group met the primary endpoint of the sUA reduction threshold compared with 39 percent of those who received Krystexxa and placebo.
There was also a significant reduction in infusion reactions with Krystexxa: four percent of patients that received Krystexxa with methotrexate experienced infusion reactions compared with 31 percent of patients who were administered Krystexxa with placebo. There were no new safety signals reported in the trial.
Related: Vtama (tapinarof) Cream Gains FDA Approval for the Treatment of Plaque Psoriasis in Adults
“The approval for Krystexxa with methotrexate is the culmination of more than five years of effort and demonstrates Horizon’s commitment to working together with the gout community to improve the patient experience and outcomes,” Horizon Therapeutics research and development executive vice president Elizabeth Thompson said.
“Immunomodulatory therapies like methotrexate are often used with biologics to reduce the development of anti-drug antibodies and allow more patients to achieve a complete response.”
Since acquiring Krystexxa from Crealta in 2015 in a deal worth $510 million, Horizon has focused on developing the drug for patients with more severe and therapy-resistant gout.
Gout is a significant therapeutic area for Horizon. The company has partnered with the likes of HemoShear Therapeutics to discover new gout therapies, as well as Arrowhead Pharmaceuticals to develop an RNA interference (RNAi) therapeutic for the disease. With Krystexxa, Horizon is looking for ways to improve the patient experience, such as by reducing infusion times from twice a month to once a month.
The drug has proven to be quite profitable for Horizon as it hit a record of $565.5 million in net sales last year, which is a year-over-year growth of 39 percent. It is projected to grow over 20 percent this year.











Join or login to leave a comment
JOIN LOGIN