If generic medications have one thing going for them it’s that they’re usually cheaper compared to their branded counterparts. This presents an obvious cost-saving benefit to patients and payers, and the generics companies themselves are able to profit from the sale of a popular drug without having to shell out the billions of dollars necessary to get a product from conception to market.
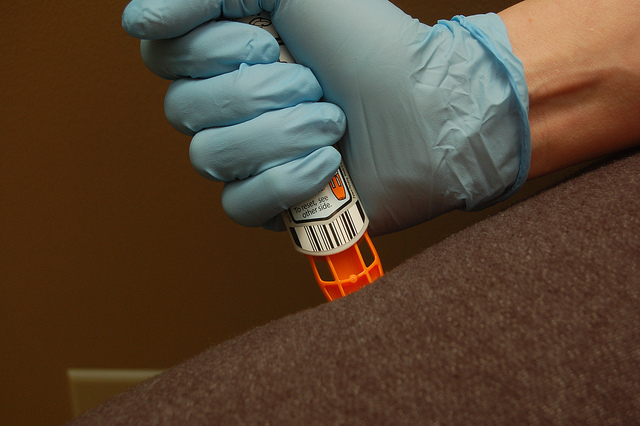
So it came as a surprise when Teva Pharmaceuticals announced last week that the list price of its generic EpiPen device would be set at $300 – the same cost as the Mylan’s own authorized generic version of the auto-injector. Without undercutting the price of Mylan’s own device – which was launched at a 50 percent discount nearly two years ago in December of 2016 in response to growing criticism of Mylan’s price hikes on its branded EpiPen – what incentive will pharmacists have to substitute Teva’s version when filling a patient’s prescription?
The answer may be simple: supply. 2018 has been a hard year for Mylan with ongoing manufacturing issues at a Pfizer-owned Meridian Medical plant affecting the company’s ability to meet demand for the EpiPen in the US, Canada and other countries. Mylan’s last update on the EpiPen shortage came in August when the company announced it was “actively exploring several options with Pfizer that would help stabilize supply.”
Teva’s generic EpiPen was approved in August of this year as the first unbranded version of the auto-injector to be offered in the US. If Teva is able to work with its manufacturing partner, Antares Pharma, to ensure consistent and reliable production of the combination device, it might not need to beat Mylan in terms of price in order to gain market share.
RELATED: Teva’s Generic EpiPen Approved by FDA Just in Time for Back-to-School
A representative from Teva was not immediately available for comment.
But while Teva’s generic EpiPen will be hitting pharmacy shelves soon, the company warned of the “release of limited doses” with additional supply to follow in the New Year. In addition, the 0.15 mg version of the epinephrine injection – a generic of Mylan’s EpiPen Jr device – likely also won’t be available until 2019.
“We’re pleased to provide access to epinephrine injection (auto-injector) for patients who may experience life-threatening allergic emergencies and we’re fully dedicated toward ensuring additional supply in 2019,” said Brendan O’Grady, EVP and Head of North America Commercial at Teva.
Teva isn’t the only generic company looking to get into the lucrative epinephrine autoinjector market. Earlier this year, Novartis’ generics unit, Sandoz, announced it would be partnering with Adamis Pharmaceuticals to commercialize its EpiPen rival, Symjepi. The FDA has approved both the 0.3 mg and the 0.15 mg versions of the auto-injector, however the devices have yet to be launched on the US market.
Kaléo’s AUVI-Q device is another EpiPen alternative for patients in the US and Canada with severe allergies, with the company recently reaffirming the availability of its device in the face of the ongoing EpiPen shortage. Last year, CVS pharmacies also began selling a cheaper alternative to Mylan’s EpiPen: the Adrenaclick.
In August of this year, the FDA took the unprecedented step of extending the expiry date of specific lots of Mylan’s EpiPen in hopes this could help patients bridge the gap before getting their next prescription filled. Considering that there’s no end in sight to Mylan’s supply issues for the EpiPen, the imminent availability of Teva’s generic version can only be a good thing for the Israeli drugmaker and for patients at risk of severe anaphylaxis.








Join or login to leave a comment
JOIN LOGIN